Diabetes mellitus (DM) is a metabolic disease caused by insulin resistance or reduced insulin secretion with the end result of persistent hyperglycaemia. The prevalence of DM is estimated to be one in 170 cats in the UK (Panciera et al, 1990; O'Neill et al, 2016). This is likely to be due to a greater prevalence of obesity in cats, increased longevity and indoor confinement (Scarlett and Donoghue, 1998; Slingerland et al, 2009; Courcier et al, 2010). Other risk factors include being neutered and certain breeds have a higher risk, such as Burmese cats (Rand et al, 1997). Male gender has been reported to be a risk factor in some but not all studies (O'Neill et al, 2016).
Aetiopathogenesis
Insulin is synthesised in pancreatic β-cells. Its effects can be considered in terms of length of effect as short, intermediate and long term. The short-term effects are to lower blood glucose by increasing glucose uptake by cells (predominantly muscle and adipose tissue). Insulin also increases amino acid, potassium and phosphate uptake into cells. The stimulus for insulin release is high blood glucose, and insulin release is augmented when there is concurrent increased amino acid and fatty acid concentration in the blood from food digestion. The intermediate effects of insulin are to inhibit the breakdown of fats from adipose tissues. The long-term effects are predominantly anabolic, promoting the uptake of amino acids and increasing protein synthesis in muscle, liver and adipose tissue, and inhibiting skeletal muscle breakdown (Dimitriadis et al, 2011), and maintaining or increasing muscle mass.
The most common cause of DM in cats is a combination of insulin resistance and reduced insulin secretion due to pancreatic β-cell dysfunction, which is similar to type 2 diabetes in people (Nelson and Reusch, 2014; Gilor et al, 2016). This may lead to reduced β-cell numbers and an inability to secrete sufficient amounts of insulin to maintain normal blood glucose; this process is progressive unless treated (Zini et al, 2009).
A substantial proportion of cats develop DM as a consequence of insulin resistance caused by endocrine diseases (e.g. acromegaly or hyperadrenocorticism) or administration of insulin-antagonistic drugs (e.g. prednisolone), or as a consequence of pancreatitis (Nelson et al, 1999; Niessen et al, 2015). Treatment of the underlying condition causing the insulin resistance or withdrawal of insulin-antagonistic drugs can resolve this type of DM.
Clinical signs are usually observed when the capacity of the kidney to resorb filtered urine glucose is exceeded, which results in glucosuria. This is likely to occur at blood glucose levels ~15 mmol/litre or higher in cats. Loss of glucose into the urine increases urinary water loss (osmotic diuresis), resulting in polyuria with compensatory polydipsia. Cats also lose weight as a consequence of reduced glucose uptake into cells for metabolism and increased loss of energy (i.e. glucose) into their urine.
Diagnosis
The triad of clinical signs — polyuria, polydipsia and weight loss — is a common reason for an owner to take a diabetic cat to the veterinary practice. The two main other differential diagnoses for this triad of clinical signs are chronic kidney disease and hyperthyroidism. If left untreated, DM can result in diabetic ketoacidosis. Ketoacidotic cats frequently have excessive urinary water loss and excessive fat breakdown, causing marked dehydration and ketone formation. These patients may present with vomiting, weakness, collapse or in cardiovascular shock.
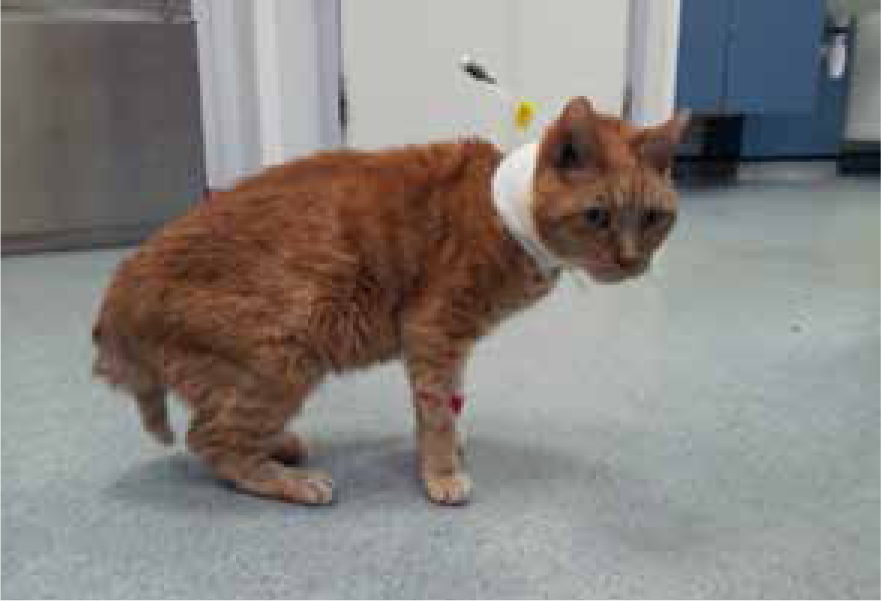
Physical examination findings vary, which in part can be attributed to the chronicity and severity of undiagnosed DM. Most cats are subdued, have a coarse, dry coat and lumbar muscle mass loss. Some diabetic cats present with generalised weakness while others may have a plantigrade stance (standing on their hocks) caused by a combination of factors, including weakness, hypokalaemia and concurrent nerve degeneration caused by diabetic neuropathy (Figure 1). Similar to dogs, cats can develop cataracts, but these lens opacities are not normally identifiable without close ocular examination. Hepatomegaly secondary to liver lipid and glycogen deposition may be palpable during abdominal palpation.
A diagnosis of DM is made if there is evidence of chronic hyperglycaemia. This is achieved when cats have the typical history and clinical examination findings caused by hyperglycaemia as described above and laboratory tests support chronic hyperglycaemia. There is no current consensus of blood glucose value for the diagnosis of DM in cats, but chronic hyperglycaemia that exceeds the kidney glucose threshold is generally accepted (>15 mmol/litre — a normal level is 4.1–6.5 mmol/litre) (Reeve-Johnson et al, 2016). Spot glucose values are unreliable in cats because their predisposition to stress hyperglycaemia can result in glucosuria if >15 mmol/litre (Rand et al, 2002). This stress hyperglycaemia also reduces the reliability of urine glucose as a sole diagnostic test for DM in cats.
The combination of consistent clinical signs, hyperglycaemia and glucosuria are very suggestive of DM. Serum fructosamine is a measurement of plasma proteins that are bound to sugars such as glucose, and is considered to be a surrogate marker of average blood glucose levels over the preceding 7–14 days (Link and Rand, 2008). Fructosamine measurements above the reference interval of the laboratory also support a diagnosis of DM.
Concurrent conditions
There are several other tests to include in the work-up for DM diagnosis in cats. A complete blood cell (CBC) count and serum biochemistry can be useful to identify concurrent conditions such as chronic kidney disease. Measurement of total T4 should be considered as there is considerable overlap between the clinical signs of DM and hyperthyroidism.
Urine bacterial culture should be considered because around one in eight cats have a urinary tract infection at the time of DM diagnosis (Bailiff et al, 2006). It is also important to measure urine ketones at the time of presentation with suspected DM (Figure 2). The urine ketone identified on urine dipsticks is acetoacetate and urine ketones should resolve if present at diagnosis after insulin therapy is started. A newly diagnosed inappetent diabetic cat will typically need hospitalisation and intensive initial therapy, and measurement of urine ketones is an important aid to identify patients that are in diabetic ketoacidosis.

There appears to be a high prevalence of exocrine pancreatic disease in cats with DM, with reports of 72% of patients affected in postmortem studies and up to 83% of diabetic cats having increased feline pancreatic lipase immunoreactivity (fPLI) (Goossens et al, 1998; Forcada et al, 2008). Interestingly, no association has been found between the presence of pancreatic changes at postmortem or fPLI levels and diabetic control in these studies. Any increase of fPLI will need to be taken into consideration with the clinical signs of the patient.
Up to 25% of cats in the UK may have DM secondary to acromegaly (Niessen et al, 2015). The test of choice to identify this condition is serum IGF-1 levels. The hormone IGF-1 is released by the liver in response to insulin and growth hormone. Serum IGF-1 levels >1000 ng/μl are 95% predictive of acromegaly.
Diabetic cats might have slightly higher systolic blood pressure than healthy cats but their blood pressure does not commonly require antihypertensive treatment unless the blood pressure is above 150 mmHg (Al-Ghazlat et al, 2011).
Diabetic nephropathy is a kidney disease associated with reduced kidney function and urine protein loss. Measurement of proteinuria using a urine protein:creatinine ratio after resolution of any underlying urinary tract infection could be considered to identify proteinuric cats that may benefit from medications to manage proteinuria, such as benazepril or telmisartan.
Cats might also experience neurological complications associated with DM, which are thought to occur as a consequence of nerve myelin defects or nerve degeneration. The most common clinical presentation of this neuropathy is generalised weakness, plantigrade stance and reduced tendon reflexes (Mizisin et al, 2002). These changes appear to be more likely if DM has been untreated for some time or chronically poorly controlled despite insulin therapy.
Treatment
Insulin
Almost all cats are best treated using twice-daily insulin injections. There are two licensed insulin products for use in cats in the UK: Caninsulin (MSD Animal Health, Milton Keynes); and ProZinc (Boehringer Ingelheim Vetmedica, Berkshire) (Table 1). Both Caninsulin and ProZinc are U-40 insulins, meaning they contain 40 units of insulin per ml so should only be administered using U-40 syringes; Caninsulin can also be administered using a VetPen. Caninsulin is a porcine lente insulin which has an intermediate duration of action and typically induces a nadir of blood glucose after 4 hours of administration.
| Insulin trade name | Manufacturer | Onset | Peak effect | Duration | hours | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | 22 | 24 | |||
| Caninsulin | MSD Animal Health | ||||||||||||||
| ProZinc | Boehringer Ingelheim | ||||||||||||||
| Lantus | Sanofi | ||||||||||||||
Cats may benefit from treatment with longer-acting insulins. The recent International Society of Feline Medicine consensus on the practical management of diabetes mellitus in cats recommends the ‘use of longer acting insulin preparations … injected twice daily, for optimal diabetic control’ (Sparkes et al, 2015). The only longer acting insulin preparation licensed for the treatment of diabetes in cats in the UK is ProZinc. This is human recombinant protamine zinc insulin (PZI), which has a duration of action of 10–14 hours.
Glargine (Lantus, Sanofi-Aventis, Fawdon) is another long-acting insulin but is not licensed for use in cats in the UK (Table 1). This insulin forms microcrystalline structures that dissolve gradually when injected subcutaneously. The advantage of this type of insulin is that the glucose nadir and peaks are less marked than with shorter-acting insulins and its use is associated with fewer resulting clinical hypoglycaemic events (Roomp and Rand, 2013; 2016). Glargine is a U-100 insulin type that can be purchased as vials or in a pen.
Regardless of the type of insulin used, it is important to remember that the response to insulin varies from cat to cat and glucose monitoring is recommended to determine the dose required (Figure 3).

Diet
The chance of diabetic remission increases and diabetic control improves when a low-carbohydrate diet is used as part of a treatment protocol that includes the use of a longer-acting insulin and close blood glucose monitoring (Gottlieb and Rand, 2013). Although postprandial hyperglycaemia is unpredictable in cats and may occur up to 10 hours after feeding, it is lower in cats fed a low-carbohydrate diet than in those that are given higher carbohydrate diets (Singh et al, 2015). The authors' recommendation is to feed low-carbohydrate diets, preferably wet food, unless there is a concurrent condition that is known to be improved by feeding a particular diet, i.e. chronic kidney disease or food-responsive enteropathy or inflammatory bowel disease.
Obesity is associated with insulin resistance (Appleton et al, 2001). It is important to achieve an optimal body condition of any obese diabetic cat to aid glycaemic control. It is also important that weight loss is controlled and monitored rather than allowed to occur as a consequence of poor diabetic control. Ideally, the number of calories needed should be calculated based on estimated ideal body weight (BW), and the following formula can be used: caloric need (in kcal) = 30 x BW (kg) + 70). This initial calculation is only a guide to start with and caloric intake will need to be adjusted based on frequent weight checks. Weight loss of 0.5–1% BW per week is desirable, although this might be difficult to achieve in some cats.
The optimal feeding regimen for diabetic cats has not been established, but the authors' preference is to feed twice daily at the time of insulin injections. If a cat prefers to eat its food gradually throughout the day, it is not necessary to change this feeding routine, especially if the cat is fed a low-carbohydrate diet and receiving long-acting insulin. Preferentially, cats should eat at least 25% of their meal before being given their insulin injection.
Monitoring the diabetic cat
The initial insulin dose given to a newly diagnosed diabetic cat must be a safe dose that will not cause hypoglycaemia. This is typically 0.25–0.5 units/kg given every 12 hours, but should preferably be kept at the lower end of this range; most diabetic cats (unless very large) do not require a starting dose of more than 1 U given every 12 hours. These doses are for Caninsulin, ProZinc and glargine insulin.
The decision to hospitalise a newly diagnosed stable diabetic patient at the start of insulin therapy is made by individual veterinary practices. It should be noted that the goal of this initial period of hospitalisation is not to achieve good diabetic control but to determine if the dose and frequency of insulin prescribed is safe for the patient — i.e. to ensure iatrogenic hypoglycaemia is avoided. Alternatively, cats can be discharged after owners have been informed about how to inject insulin and educated on the clinical signs of hypoglycaemia. Discussing home blood glucose monitoring can be undertaken at a later appointment once owners have adjusted to the initial management of their diabetic cat. The first re-examination appointment is typically 1 week after discharge.
Good client communication is essential as the clinical signs of diabetes (polyuria, polydipsia and polyphagia) should begin to improve after insulin therapy is started. Repeat physical examinations and bodyweight measurements are important as cats receiving treatment should begin to gain weight and not have ongoing muscle mass loss.
Laboratory tests can also be used to monitor diabetic control. Blood glucose monitoring can be performed via glucose curves or measurement of serum fructosamine (fructosamine is a marker of glycaemic control over the preceding 7–10 days).
Blood glucose curves typically require the collection of seven blood glucose values over the course of 12 hours, with the first glucose reading taken at the time of the morning insulin injection, followed by samples every 2 hours up to and including the time of the evening insulin injection. The frequency can be increased to hourly measurements if there is an area of interest in the curve; for example, hourly blood glucose measurements may be made if the blood glucose goes below 7 mmol/litre and moved back to 2 hourly after glucose has increased to >7mmol/litre. Samples can be collected from the marginal ear vein or footpads. Preferably local anaesthetic creams (e.g. EMLA cream) can be applied 45 minutes before sampling and a thin layer of petroleum jelly applied immediately before sample collection. Pressure should be applied to the venepuncture site until bleeding has stopped. A veterinary calibrated glucometer should be used to increase the reliability of glucose readings; this becomes more important toward the hypoglycaemic range.
Encouraging owners to learn how to perform blood glucose measurements at home should aid in longer-term DM management and specifically addresses a key concern of owners of diabetic cats, which is ‘wanting more control’ in one study (Niessen et al, 2010).
There is marked day-to-day variation in blood glucose curves in cats (Alt et al, 2007). This is one of the main reasons why spot glucose checks provide limited information about glycaemic control. Owners can be trained to collect blood glucose readings at home and the authors have found that 75% of owners are willing and able to perform blood glucose measurements when given direction and instruction (Gostelow et al, 2016). Home blood glucose readings should be recorded in a written diary or electronically in a spreadsheet or dedicated diabetic phone app. The free RVC Pet Diabetes App is available from the Android (http://bit.ly/1q3jCV5) and iPhone App (http://apple.co/203OoK2) stores.
| β-cells | Insullin-secreting cells within pancreatic islets of Langerhans |
| Diabetic remission | Normoglycaemia without antihyperglycaemic treatment for a minimum of 4 weeks |
| Fructosamine | Measurement of glycosylated proteins. Used as a marker of glycaemic control over the preceding 7–10 days |
| Hyperglycaemia | Blood glucose above the laboratory reference interval |
| Hypoglycaemia | Blood glucose below the laboratory reference interval |
| Iatrogenic hypoglycaemia | Medically induced hypoglycaemia, which occurs as a consequence of insulin administration |
| Ketones | This refers to three molecules: acetone; acetoacetate; and 3-β-hydroxybutyrate. They are formed in the liver during fatty acid metabolism |
| Nadir | Lowest blood glucose level caused by insulin administration |
| Plantigrade stance | Standing on the hock/tarsus |
It is essential to educate owners about the risks of iatrogenic hypoglycaemia when patients are discharged. The initial signs of hypoglycaemia in cats can be subtle, ranging from lethargy, weakness and vocalisation to tremors, ataxia and, later, seizures and coma if hypoglycaemia is not corrected. Owners should administer a rapidly acting source of carbohydrate such as GlucoGel or honey. The rapidly acting source of carbohydrate should normalise blood glucose and is often an effective treatment for hypoglycaemia. Food should then be given to sustain normoglycaemia. Owners should then contact the veterinary practice for further advice.
There are many situations in which owners question whether their cat could be experiencing hypoglycaemia or just behaving differently. It these situations, home measurement of blood glucose can be useful. This allows owners to identify the glycaemic status of their pet, which will mean informed decisions can be made — an owner will know if a cat is hypoglycaemic and should be given glucose supplementation.
A fructosamine measurement should be performed at time of diagnosis. As a general recommendation, all diabetic cats should be re-examined after 1 week of starting insulin therapy. A repeat fructosamine measurement can be made at this time to monitor if there has been any improvement in the cat's glycaemic control. Alternatively, if the cat's clinical signs are improving, then fructosamine measurements can be reserved for when there is concern over whether a patient's condition is deteriorating as a consequence of worsening diabetic control or due to another concurrent condition. For example, a cat has an increase in thirst and the veterinarian wishes to determine if this is due to uncontrolled DM or another polydipsic condition such as chronic kidney disease. One word of caution is that fructosamine is a measurement of glycated proteins — therefore fructosamine is only a reliable indicator of glycaemic control if cats have normal blood protein levels (albumin and globulins) and do not have uncontrolled hyperthyroidism.
Urine glucose as a surrogate marker of blood glucose levels has limited utility. However, there is one situation where measurement of urine glucose is useful, which is when glucosuria is persistently absent in a cat receiving insulin treatment. This patient could be receiving too much insulin or entering diabetic remission. Further information is required to determine the cause, such as home blood glucose measurements. Urine samples can be used to measure urine ketones. The development of urine ketones is a marker of worsening DM control, and the persistence of urine ketones suggests persistently inadequate control. These patients are likely to need close monitoring to determine if they require an insulin dose increase or should be given a longer acting insulin. Again, a blood glucose curve can aid in this decision making.
Some cats do not tolerate repeated needlesticks for glucose monitoring. There are two possible options for these patients. One is to use clinical signs and fructosamine measurements to guide management. The issue with this method is that the length of action of insulin or glucose nadir cannot be estimated; fructosamine value is only a marker of the quality of diabetic control and the reason for poor diabetic control, such as short duration of insulin action, cannot be established using this parameter.
An alternate method is to use interstitial glucose monitoring. This involves using subcutaneous catheter-like monitors that connect to a transmitter and send glucose data wirelessly to a monitor. These monitors provide interstitial glucose measurements which are a close approximation of blood glucose levels, provide measurements every 5 minutes and can remain in place for up to 6 days. However, they need calibrating with actual blood glucose measurements from the cat every 12 hours and there is a learning curve when starting to use these kits. ‘Bloodless’ glucose meters may be available in the future as technologies are in development for human diabetics, such as GlucoWise, and it will be interesting to find out if these can be used in cats.
Other treatment options
Many owners will enquire about alternatives to insulin or treatments that can be used in conjunction with insulin to manage their cat's DM. Insulin-sensitising drugs are commonly used in the management of human DM, and one of the first line drugs used in humans is metformin. This has been trialled in cats but often causes gastrointestinal upset and lethargy and is therefore not commonly used (Nelson et al, 2004).
Other insulin-sensitising drugs include glipizide, pioglitazone and exenatide. Glipizide is an oral medication that stimulates endogenous pancreatic insulin release in addition to improving insulin sensitivity. It is probably the oral drug of choice if owners are unable or unwilling to administer insulin injections. However, this drug may cause long-term pancreatic damage and is effective in only a small proportion of diabetic cats (Feldman et al, 1997; Goossens et al, 1998; Hoenig et al, 2000). Glipizide is also not suitable for cats in diabetic ketoacidosis. Pioglitazone has been shown to increase insulin sensitivity in obese cats but has not yet been reported in diabetic cats (Clark et al, 2014). Exenatide is an injectable drug that increases endogenous insulin secretion and protects the pancreatic islets from DM-induced damage. It appears to be safe to use in cats and further research is ongoing to determine if it will be beneficial in the management of diabetic cats (Riederer et al, 2016).
Other treatments that have been used in small numbers of cats are intestinal glucose absorption inhibitors (acarbose), and chromium tripicolinate, which was thought to improve insulin sensitivity (Appleton et al, 2002). However, neither of these therapies have been proven to be effective in large numbers of cats and are currently considered to have limited usefulness for diabetic cats.
It should not be forgotten that treating concurrent diseases in diabetic cats can improve glycaemic control. Urinary tract infections, skin infections, dental disease and hyperthyroidism can all contribute to insulin resistance in difficult to control diabetes.
It is more difficult to prove mild to moderate chronic pancreatitis in diabetic cats but, if a cat is frequently becoming inappetent, then consideration of a novel protein diet trial and measurement of serum vitamin B12 can be considered.
Advice and nurse clinics
A strong nurse-owner bond is very important in the management of diabetic cats. A team approach will often lead to better outcomes. Humans receive better diabetic care at practices that employ specific nurse practitioners than at physician-only practices, and specialist diabetic nurses have been described as the ‘lynchpins of quality diabetic care’ (Ohman-Strickland, 2008). This is likely to be the case in veterinary medicine.
Veterinary nurses have a key role in the education of owners of diabetic cats. Owners should have a good knowledge of the disease and good practical skills to manage their cat at home. This can be achieved during nurse consultations and by directing owners of diabetic cats to responsible websites with videos that can help them better understand the disease and improve their treatment techniques (e.g. www.facebook.com/RVC.Diabetic.Remission.Clinic).
Nurse appointments are good opportunities to check if owners have enough consumables at home such as insulin needles, a sharps box and glucometer equipment. Documenting the weight of the cat at every in-practice nurse appointment, checking if owners are comfortable with injection technique — which may include asking them to inject their cat with sterile water during the consultation — and checking that owners store insulin appropriately are all functions of a nurse clinic. These in-practice clinics can also incorporate blood pressure monitoring, a free catch urinalysis check and physical examinations such as dental checks. Veterinary nurses should be able to interpret blood glucose curves; blood glucose values <3 mmol/litre should be reported to the veterinary surgeon because the insulin dose will need to be reduced. Nurses should be able to determine if a cat's DM is well controlled or if an appointment with the veterinary surgeon is needed.
A good diabetic veterinary nurse should aim to find out what is normal for each diabetic cat and what each owner is capable of. This can be achieved by in-practice and telephone nurse clinics and email communications. Having a point of contact is reassuring for owners, particularly in practices where the veterinary surgeon may work at several branches and owners have trouble contacting them or see different veterinarians at each appointment.
Owners may be more likely to confide in a veterinary nurse over how their cat's DM is making them feel, how they are coping with the routines of DM management and any concerns they may have. Owners of diabetic cats have reported to the authors that feeding their diabetic cat a diet that is different from other cats’ food in the household can be challenging. One option is to use microchip-activated feeders such as SureFeed Microchip Pet Feeder (SureFlap, Cambridge).
Another area of concern for owners is who will look after their cat while they are away on holiday, and having a list of catteries capable of managing a diabetic cat or being able to board diabetic cats in the practice is useful for owners (Niessen et al, 2010).
Nurses can provide telephone advice to concerned owners of cats with DM. If there is concern about hypoglycaemia, owners can be instructed how to best manage this situation. A common question is how much insulin to give when a cat is inappetent but otherwise stable. It is often safe to recommend giving half the usual dose of insulin; if a cat normally receives two units twice daily, it can be given one unit twice daily, but the cat should be examined if its appetite does not return after being given the half doses. It is good practice to examine a cat sooner if it is unwell. If there are no appointments for an hour or 2 or before closure that day, an in-practice nurse appointment can be scheduled as soon as possible, and certainly the same day, to triage the cat, assess current glucose status and make the consulting veterinarian aware of the condition of the cat following the initial triage.
Diabetic remission
Diabetic remission is defined as normoglycaemia without antihyperglycaemic treatment for a minimum of 4 weeks (Gostelow et al, 2014). Diabetic remission rates vary between studies from 11% up to 100%, which in part can be explained by different diabetic protocols and different populations of cats used for these studies. Remission is possible because hyperglycaemia is directly toxic to pancreatic islets and sustained normoglycaemia or excellent diabetic control can allow reversal of β-cell dysfunction (but not reversal of β-cell loss) (Zini et al, 2009).
Known positive prognostic factors for the potential of diabetic remission are good glycaemic control within 3 weeks of diagnosis, lack of diabetic neuropathy, lack of hypercholesterolaemia, DM diagnosis in older cats and treatment with steroids before DM diagnosis. These prognostic factors can be summarised as: cats not having signs or blood tests results consistent with late diagnosis; achieving good early control; and removal of underlying trigger factors.
Diabetic remission can be achieved after several months to a year of insulin treatment. Owners should be aware their cat may become increasingly insulin sensitive over time and DM in cats is uncommonly a static condition, particularly in the first year of treatment.
Cats that achieve diabetic remission may not remain insulin free for life, with reports that around 25–30% of cats in remission later relapse, typically within 12 months (Zini et al, 2010; Gottlieb et al, 2015). This is because glucose tolerance may not become normal despite achieving normoglycaemia and cats that have fasting blood glucose levels >7.5 mmol/litre have an increased risk of relapse within a year (Gottlieb et al, 2015). Owners should be aware of this potential for relapse and monitor their cat for recurrence of clinical signs of DM. To reduce the risk of relapse in these cats, it is important to continue feeding them a low carbohydrate, wet food diet if possible, maintain good body condition and treat any concurrent conditions, including dental disease.
Conclusion
There are many challenges in managing a diabetic cat. Understanding the disease pathogenesis allows improved management.
Diabetic cats fall on a spectrum of disease, with some having mild to moderate β-cell dysfunction at diagnosis while others have permanent β-cell loss.
Identifying and treating concurrent diseases that can cause insulin resistance should make management easier, although some cats remain challenging despite this.
The veterinary nurse has a critical role in the management of the diabetic cat. This involves owner education and providing advice. They should also be able to identify simple and complex problems and oversee diabetic inpatient cats that are undergoing diabetic monitoring and healthcare procedures.

