The patient presented to the referring veterinary surgeon (RVet) after vomiting and collapse during a walk with their owner. The owner reported that the patient had struggled to keep up with them and that they turned around to see the patient staggering. Shortly afterwards the patient produced yellow, bile-tinged vomitus and collapsed. In this state the patient was unresponsive to both touch and sound. The patient presented to the RVet with ataxia, pale mucous membrane (MM) colour and a temperature of 39.3°C. There was no known disease prior to hospitalisation.
Signalment
Species: Canine
Breed: Labrador retriever
Age: 9 years
Sex: Female (neutered)
Weight: 30.5 kg
Patient assessment
The RVet conducted thoracic and abdominal radiographs which revealed a fluid build-up around the kidneys and unremarkable thoracic images. Ultrasound was performed and free fluid was identified in the splenic region. The patient received intravenous fluid therapy (IVFT) and a blood sample was taken for haematology and biochemistry tests. Biochemistry revealed mildly elevated creatinine and mild hypoglycaemia, while haematology showed band neutrophils and reticulocytes were present (Table 1). Electrocardiography (ECG) revealed very high T-waves with tachycardia of 148 beats per minute (BPM) (Table 2). On discussion with the owner, the patient was referred to an intensive care unit.
Table 1. Patient blood test results
| Blood Biochemistry reference ranges: | ||||
|---|---|---|---|---|
| Sample type blood | Reference interval | |||
| Urea | * | 9.9 | mmol/l | 2.0–7.0 |
| Creatinine | * | 97 | µmol/l | 100–133 |
| Total protein | * | 44.7 | g/l | 63.0–71.0 |
| Albumin | * | 20.4 | g/l | 32.0–38.0 |
| Globulin | * | 24.3 | g/l | 20.0–35.0 |
| Albumin/globulin ratio | * | 0.84 | 0.60–1.50 | |
| Alanine aminotransferase | * | 9309 | IU/I | 20–60 |
| Alkaline bilirubin | * | 234 | IU/I | 0–110 |
| Total bilirubin | * | 38.6 | µmol/l | 0.0–10.0 |
| Aspartate | * | 1171 | IU/I | 20–35 |
| Phosphate | * | N/A | mmol/l | 0.75–1.25 |
| Cholesterol | * | 2.2 | mmol/l | 3.5–7.0 |
Film comments Acanthocytes **, Schistocytes **, Target cells *, Elliptocyes *, Microcytic red cells *, Thrombocytopenia. H= High and L= Low
Table 2. Normal parameters in canine patients
| Heart rate: | 60–140 beats per minute |
| Respiratory rate: | 10–30 breaths per minute |
| Temperature: | 38.0–38.5°C |
| Mean arterial blood pressure: | 85–120 mmHg |
| Systolic blood pressure: | 110–160 mmHg |
| Diastolic blood pressure: | 60–90 mmHg |
| Packed cell volume: | 48–66% |
Patient history
During examination the patient was in a collapsed state with a heart rate (HR) of 160 BPM, respiratory rate (RR) of 32 breaths per minute, temperature of 38.7°C, pink MMs and absent peripheral pulses. Thoracic auscultation and abdominal palpation were unremarkable. The clinician proposed that the patient had ingested xylitol, a sugar alcohol commonly used as a sweetener (Dunayer, 2006), resulting in primary acute hepatopathy and secondary coagulopathy. However, the patient's symptoms were also synonymous with leptospirosis and so barrier nursing was requested.
The mechanism behind xylitol-induced liver necrosis is still unclear however it has been suggested that xylitol and its metabolites cause the depletion of adenosine triphosphate within hepatic cells leading to cell death. An alternative mechanism involving the production of reactive oxygen species has also been proposed (Bailey, 1998). This proposed mechanism suggests that xylitol ingestion leads to the generation of free-radical oxygen species within the liver which cause fatal, oxidative damage to hepatocytes.
Once admitted, the patient received a 2 ml/kg/hour infusion of Hartmann's solution (compound sodium lactate, Dechra) to maintain hydration and electrolyte balance. Fresh plasma (Pet Blood Bank) was infused at 6 ml/kg/hour over 12 hours to correct clotting factor deficiencies. The patient received a 5 ml/kg bolus of Geloplasma (modified liquid gelatin, Fresenius Kabi) to promote the restoration of normovolaemia, normotension and tissue perfusion. Prior to admission, the patient had received a 1 mg/kg dose of the anti-emetic Cerenia® (maropitant, Zoetis) intravenously (IV), a 10 mg/kg dose of the antibiotic Co-amoxiclav (augmentin-clavulanate, GlaxoSmithKline) IV to combat potential Leptospira bacteria and the proton pump-inhibitor Zantac® (ranitidine, GlaxoSmithKline) had also been given IV to reduce the risk of stress-induced gastric ulceration which is commonly seen in critically ill patients (GlaxoSmithKline Inc., 2015). Finally, the patient was prescribed a 5 mg/kg dose of vitamin K1 (Laboratoire TVM) to be given IV twice daily to help correct clotting factor deficiencies.
Case description
Patient monitoring
On admission temperature, pulse rate and respiratory rate (TPR), MM colour, capillary refill time and blood pressure were measured every 4 hours. All findings were recorded on the patient's care plan. During the night, the patient's HR fluctuated 130 BPM, RR was, on average, 28 breaths per minute and temperature was 38.7°C. The patient was hypotensive with an initial mean arterial blood pressure (MABP) of 69 mmHg. Following two blood transfusions MABP increased to 99 mmHg by 07:15 am the following morning. See Table 2 for all normal values. During transfusions the patient was monitored for signs of urticaria, shock, vomiting and fever every 15 minutes. No adverse reactions were observed.
The patient remained lethargic. An electrocardiogram (ECG) revealed electrical activity indicative of ventricular tachycardia (VT) and so ECG monitoring was continued. At 06:00 am it was noted that the patient had stood up and so was taken outside. Each time the patient was walked urine and faecal output were recorded.
At 8:00 am the patient received a constant rate infusion of Xylocaine 2%w/v (lidocaine hydrochloride, Astra) at 11 mg/kg to correct the VT. Subsequently, ECG monitoring was continued. HR and RR measurements were taken every hour while temperature and blood pressure (BP) were measured every 4 hours. Throughout the day the patient exhibited sustained tachycardia at 170 BPM with roughly 50% of ECG traces showing ventricular premature complexes at 3:00 pm. Additionally, the patient became hypotensive with an average MABP of 80 mmHg (see Table 2 for normal values). Once the patient was stabilised, TPR, BP, MM colour and capillary refill time were observed every 4 hours until discharge.
Modification of the environment
As the patient was a large breed, they were placed into a walk-in kennel which would provide the patient with adequate space to move around in when able to mobilise effectively. Increased patient mobility reduces the incidence of decubitus ulcerations, joint stiffness, muscle atrophy and atelectasis as it encourages healthy blood flow, alleviates the pressure exhibited on lung parenchyma caused by lateral recumbency and preserves muscle mass (Sherman and Olby, 2004). The floor of the kennel was lined with incontinence pads to soak up urine in the event the patient voided themselves while recumbent and to avoid subsequent urine scalding. A thick duvet was provided to increase patient comfort and to further prevent the formation of decubitus ulcers on the patient's joints.
Nutrition
The patient's recommended energy requirement (RER) was calculated so that the correct amount of food to meet this requirement could be provided. Bland, easily digestible diets such as Sensitivity Control (Royal Canin) and Gastro Intestinal (Royal Canin) and boiled chicken were individually provided to the patient. Food was left for 20 minutes and then removed if left uneaten to prevent food aversion. Commercial diets such as Pedigree (Mars) and Cesar (Mars) were offered with no success as the patient was uninterested in them. Consequently, the patient was prescribed Cerenia® to reduce nausea. Boiled chicken was then provided with similar results despite attempts to hand feed. Following this the patient was prescribed the appetite stimulant Mirtazapine 15 mg (Medreich) offlicense.
The patient's prolonged anorexia prompted the placement of a naso-oesophageal (NO) tube to ensure the patient received the daily RER and promote recovery (Box 1). An NO tube was the favoured choice of feeding tube due to the minimally invasive nature of the device when compared with percutaneous endoscopic gastrostomy tube. On the first day of tube placement the patient was fed 33% of their RER. This was done to minimise the risk of the patient developing refeeding syndrome. Refeeding syndrome occurs when the bodily glucose metabolism shifts from mobilising glucose stores such as glycogen to metabolising carbohydrates from the patient's diet when fed. The sudden metabolic shift leads to increased insulin production and subsequent increased cellular uptake of phosphate from the blood. Anorexic animals that already have reduced phosphate uptake from their diet may soon become hypophosphataemic (Sumner, 2016).
The diet Convalescence Support (CS) (Hill's) was selected due to its high palatability and energy density. Initially, the patient was fed 90 mls of CS per feed, four times a day by this method. On the second day this increased to 66% RER and so 180 mls of CS was fed per scheduled feed. From the third day onwards the patient was fed 270 mls of food via the NO tube until the patient's appetite resumed.
Before each feed small amounts of food were offered to test patient appetite. Three days after tube placement the patient ate 53 g of boiled chicken and the remaining RER was supplemented via NO tube. The following day a further 45 g of chicken was eaten and so a/d (Hill's), a solid, palatable diet designed for critically ill patients, was offered. 250g of a/d was eaten and the remainder of the RER was tube fed. Five days after NO tube placement the patient received 100% of the RER entirely per os and maintained this until discharged.
Nursing care
The intensity of nursing care delivered varied with the stability of the patient. Prescribed nursing care was recorded onto the patient's care plan with scheduled periods of patient assessment (Figure 1). If patient parameters exceeded stated limits then the patient was to be re-evaluated by a veterinary clinician. On admission nursing care consisted of regular TPR and BP checks every 15 minutes due to the possibility of reactions to fresh plasma infusion. This was then extended to every 2 hours. IVFT monitoring was continued and sling walks were conducted every 4 hours with urination or defaecation being recorded to inform the clinical team about the patient's renal and gastrointestinal function. During this time nursing staff were asked to contact a clinician if the patient's HR rose above 180 BPM, tachypnoea/dyspnoea was apparent, systolic blood pressure fell below 100 mmHg or if the patient re-entered VT. The patient deteriorated overnight and so the care plan was modified. TPR checks were conducted hourly and BP measurements were taken every 2 hours. All prescribed medications were given by the dose, route and time specified. An ECG was also set up to monitor for signs of VT. Food was offered but the patient remained anorexic despite efforts made to hand feed.
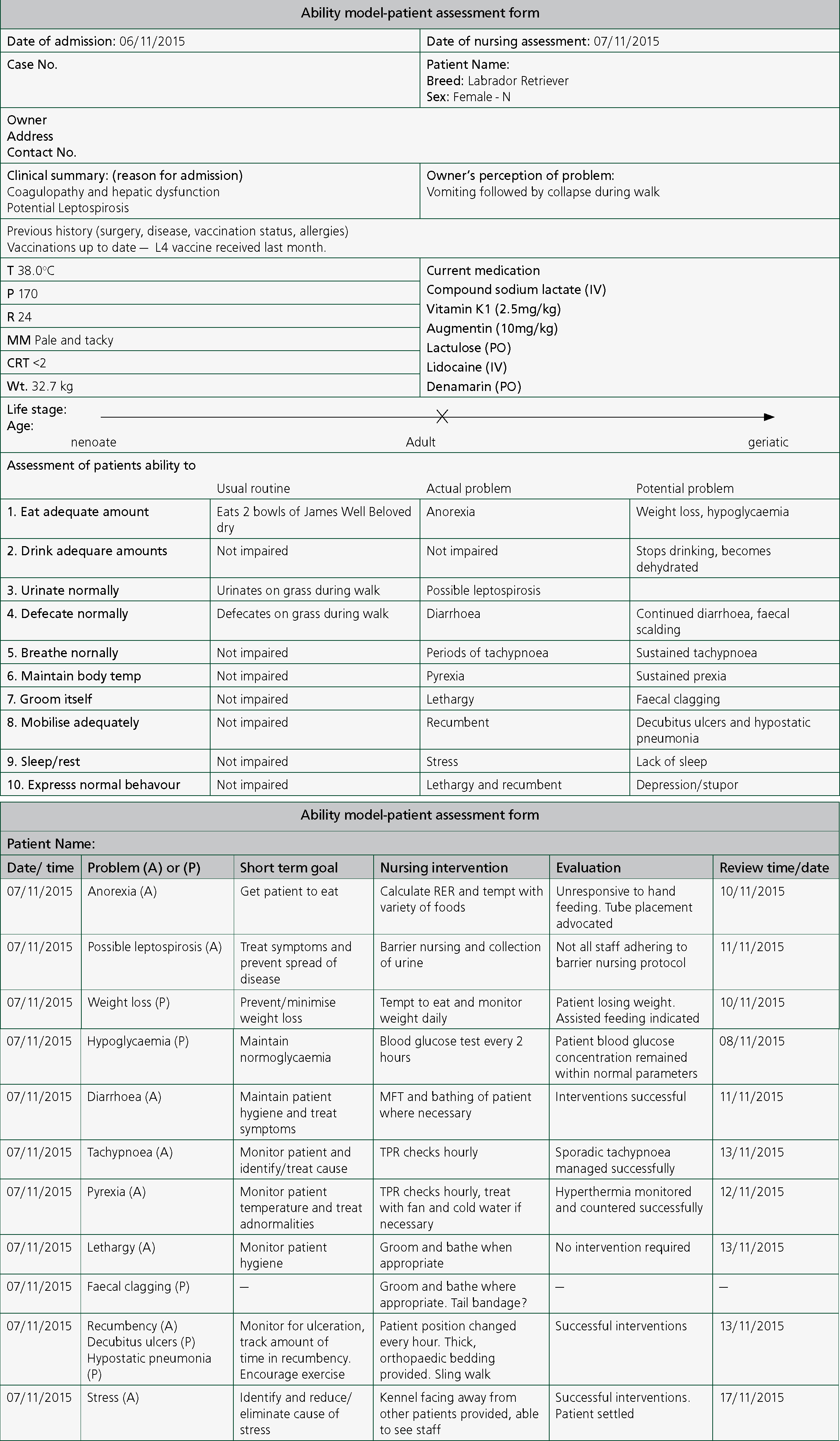
Temperature, BP and RR checks were extended to every 4 hours after the patient had stabilised and pulse rates were recorded every 2 hours. As the patient had not eaten, blood glucose measurements were recorded every 4 hours due to the predisposition of anorexic patients to hypoglycaemia. These measurements could serve as an indicator of liver function, as hepatic glycogenolysis occurs once the body enters a catabolic state. Such a state can be induced by prolonged periods of anorexia. If significant, catabolism patients rapidly lose weight once bodily protein is metabolised for energy thus resulting in cachexia. The clinician was notified after each check. The patient had repeatedly produced diarrhoea since admission and so a tail bandage was applied with barrier creams being used to prevent faecal scolding. Once per os feeding had resumed the patient was moved to the medical ward.
Diagnostics
On admission blood samples were taken. Samples were used to test prothrombin time (PT) and activated partial thromboplastin time (APTT). The patient's PT and APTT were severely prolonged, indicative of hepatopathy and coagulopathy.
Further blood tests included (Table 1):
- Serum biochemistry — liver enzyme concentrations consistent with severe hepatopathy and a bilirubin concentration indicative of cholestasis
- Haematology — revealed thrombocytopenia and mild neutrophilia
- Venous blood gas — demonstrated evidence of compensated metabolic acidosis
- Leptospira serology — positive for three species however patient had received a leptospirosis L4 vaccine (Nobivac, MSD Animal Health) the previous month
- Canine pancreatic lipase immunoreactivity — inconsistent with pancreatitis
- Cardiac troponin I — raised and consistent with myocardial inflammation.
The following day the patient underwent thoracoabdominal computed tomography (CT) scan. The scan revealed evidence of liver necrosis, ascites and changes in the pancreas. In the thorax, lesions in the right caudal lung lobe were observed. The radiology report concluded that the combination of changes witnessed were reflective of inflammatory/infectious disease or xylitol toxicity.
Long-term management
On discharge the patient was prescribed Denamarin (Nutramax Laboratories) to support the liver after acute insult. A blood sample was taken the following week for Leptospira serology as a precautionary measure and later came back as negative. The owner was advised to provide short lead walks of 5 minutes and gradually increase walk duration over 4 weeks to help build patient muscle mass without the patient over-exerting themselves. Finally, the patient was referred for hydrotherapy to further help restore muscle tone after losing 10% of bodyweight during hospitalisation. In hindsight, a comprehensive physiotherapy regimen inclusive of passive, assisted and active exercises could have been implemented to reduce weight loss during the hospitalisation period.
Discussion
On presentation the patient required urgent critical care. Prompt diagnosis improved the case's prognosis significantly. Xylitol ingestion was never confirmed but was deemed the most likely causative factor and so medical care was prescribed based on this assumption.
Blood tests on samples proved invaluable in the diagnosis of this case. The patient's serum biochemistry revealed elevated alkaline phosphatase (ALKP) and alanine transaminase (ALT) liver enzymes which were strong indicators of severe hepatopathy. Haematology was carried out as specific cell counts, such as neutrophils, informed the clinician whether an infection was present and platelet count gave insight into the patient's ability to form clots.
PT and APTT tests were performed to evaluate the effects of hepatopathy on the patient's ability to coagulate. PT is commonly used to assess the extrinsic pathway of the clotting cascade and determine the blood's ability to clot, identify signs of liver damage and assess vitamin K levels in the blood; while the APTT is used to measure the intrinsic and common pathways of the clotting cascade which convert prothrombin to thrombin in order to form a fibrin clot. Both PT and APTT were prolonged, these results demonstrated that the patient was suffering from impaired clotting function and were therefore indicative of liver damage (Badylak and Van Vleet, 1981).
A CT scan was performed to confirm the extent of damage to the liver and gain insight into the effects the condition was having on the patient's body and allow the clinician to formulate a medical management plan.
When nursing this case it was understood the patient would be recumbent for lengthy periods of time and so efforts were made to keep the accommodation comfortable by lining it with thick bedding. Ideally, an isolated kennel easily visible from the nurse's station and facing away from the other patients would have been used to help improve patient monitoring, reduce patient stress and reduce the risk of spreading potential leptospira bacteria to other patients. Unfortunately, no such facility was available and so a ‘strictly barrier nursing’ sign was placed on the patient's kennel and it was agreed with colleagues that the author and another nurse would be the primary carers for the patient to help reduce the risk of pathogen spread.
When interacting with the patient the author washed his hands thoroughly using the World Health Organisation hand washing technique (Kilpatrick, 2013) before and afterwards. Additionally, the author donned personal protective equipment (PPE) to protect himself from potential zoonotic Leptospira bacteria (WHO, 2013). The PPE provided was minimal and consisted of disposable gloves and aprons. This aspect of care could have been improved by the provision of shoe covers and facial masks as well as a foot bath to disinfect shoes in after interaction (Monsey, 2013). Additionally, the patient's urine should have been collected in kidney dishes or soaked in absorbent materials rather than allowing them to freely urinate in outside spaces shared by other patients. This is because leptospirosis can be transmitted via the urine of infected animals (Gear, 2013).
During the patient's hospitalisation the author consistently made conscious efforts to provide mental stimulation and reduce patient stress as stress can cause immune suppression and make staff–patient interactions more difficult (Hewson, 2014). When performing checks and administering medications the author would get inside the kennel with the patient, while wearing an apron and gloves to barrier nurse, sit down, talk to them and stroke them to alleviate stress. This approach proved effective when performing tube feeds. Each feed was administered over 20 minutes, this meant that the patient became comfortable with the author's presence and was therefore less stressed.
During NO tube placement care was taken to follow the manufacturer's instructions (Box 1). When applying Xylocaine 2%w/v, to produce local anaesthesia in the nasal cavity, the author comforted the patient and used minimal restraint. After waiting 15 minutes for the lidocaine to take effect (Lemke and Dawson, 2000), the NO tube was placed. When advancing the tube, no resistance was encountered. Nonetheless, the author monitored for epistaxis and sounds of crepitus. The tube was secured to the side of the patient's face using skin staples. To improve placement technique further an x-ray could have been performed to confirm tube position.
Box 1.Naso-oesophageal feeding tube placement procedureTube placement
- Assemble all equipment required:
- Device for analgesic administration to the selected nostril
- Disposable gloves
- If required a lubricant or stylet
- Feeding tube of feeding tube with stylet (for size and material selection, refer to doctor prescriptions)
- Inform the patient (if this is possible)
- Placement of the tube
- For oral or nasal placement, measure the length of tube to insert according to standard protocols
- Lubricate the feeding tube
- Hold the tube 10 cm from the end and roll the tube between the passage and move the tube carefully to the nostril posterior or throat
- Continue the careful progress of the tube until it is in pharynx, oriented towards the oesophagus
- Slowly proceed to avoid causing the patient to vomit
- Incline the head forward to close the trachea and open the esophagus. Advance the tube into the oesophagus
- Once the mark is reached, stop the advancement of the tube
- Fixation of the tube
- After preparing the skin, place an adhesive waterproof dressing. Dress over the tube at the base of the nose and apply adhesive strips on the nose. Ensure limited length of strips on patient's cheek and avoid large hoops (a potential source of blocking vision and a risk of detachment)
- Verification of position
- Preferably by x-ray
- If using a feeding tube with stylet, then remove gently the stylet from the feeding tube
- The placement of the tube is confirmed once a day and systematically before each use (in any case follow instructional protocol).
Before feeding the patient's daily RER was calculated, the feed was constituted and the position of the NO tube confirmed. Wearing gloves, the tube was wiped with antiseptic wipes to reduce risk of nosocomial infection from pathogen growth in the tube. Using a syringe, the author tested for negative pressure to confirm its position. If negative pressure was absent it would be indicative of incorrect positioning and feeding would not start (Ettinger and Feldman, 2010). Additionally, the tube was flushed with 5 mls of sterile water before and after feeding to maintain patency (Aldridge and O'Dwyer, 2013). The tube's syringe port was only compatible with syringes from the same manufacturer, this acted as a safeguard to ensure that only food was passed down the tube.
The author performed patient checks and delivered medications in accordance with the times scheduled on the patient's care plan. Adherence to care plans has been shown to help in the identification of patient deterioration, maintenance of analgesia and prevention of nausea (Brown, 2012) and so improved the patient's prognosis.
Conclusion
The patient was discharged 1 week after admission for convalescent care at home despite an initially guarded prognosis. This serves as testament to the quality of care provided to the patient during their hospitalisation. However, this is not to say that there is no room for improvement.
NO tube management proved successful in the provision of the patient's RER and the prevention of complications commonly seen in the use of these tubes. Such complications include blockage, premature removal and aspiration pneumonia (Bartges and Seim, 2003). Per os medications were delivered in the patient's tube feeds. Blockage did not occur however it is poor practice to medicate patients in this fashion due to an increased incidence of complications (Aldridge and O'Dwyer, 2013) and so should be not be repeated in future cases requiring assisted feeding.
The majority of staff followed barrier nursing protocol during the management of this case. However, some members of staff failed to adhere to this protocol prior to the return of Leptospira serology results. An inconsistent barrier nursing regimen could have resulted in the nosocomial infection of other patients or the zoonotic infection of staff (WHO, 2013) and so all protocols regarding the treatment of infectious patients should be strictly followed in the future.
The author's nursing skills were developed considerably during the management of this case. He was able to use theoretical knowledge of assisted feeding and apply it in a practical manner. The range of diagnostic procedures performed has been beneficial in expanding clinical experience and has demonstrated their importance in the division of bespoke care. If presented with a similar case in the future the author would recognise the reasoning behind the clinical decisions being made and so deliver improved nursing care in the future.
Key Points
- Registered veterinary nurses (RVNs) play a critical role in the management of acute hepatopathy. It is therefore of vital importance that they understand the potential secondary complications associated with liver damage and their treatments.
- Extensive multi-parameter monitoring should be conducted routinely or even continuously where possible, i.e ECG. All members of the clinical team must be aware of any monitoring outcomes or values which necessitate veterinary intervention.
- Nutritional support is key to a successful patient outcome. Feeding tube placement should be reserved as a last resort after several different diets, including the patient's home diet, have been trialled. If a feeding tube is placed correct tube management procedures must be strictly adhered to and efforts to resume feeding per os should be made prior to each feed.
- Formulating a patient care plan using a recognised nursing care model will improve communication between members of the clinical team and help ensure nursing care is consistent between different members of the team.
- Strict barrier nursing protocols must be implemented and closely followed until the differential diagnoses that warrant barrier nursing, such as leptospirosis, have been eliminated. Adherence to such protocols will protect the patient, the clinical team and other hospitalised patients from nosocomial disease transmission.

