This patient care report hhighlights the veterinary nursing interventions provided to a canine patient that presented to the practice with ibuprofen toxicity.
Signalment
Species: Canine
Breed: Australian Shepherd
Age: 7 months old
Sex: Male (neutered)
Weight: 16.2 kg
History
Approximately 20–60 minutes after ingesting 22–24 200 mg (296 mg/kg) ibuprofen tablets, the patient presented after hours to an emergency clinic with vomiting and an altered depressed demeanour.
Patient assessment
On examination the patient's heart rate (HR) was at the higher half of the normal range at 132 beats per minute (bpm) (normal range 80–160 bpm (Bassert and Thomas, 2013)). During palpation of the abdomen tenderness was noted in the cranial region. These were the only abnormalities found during the full body examination.
Studies have shown toxic doses start at 50 mg/kg of ibuprofen, with renal damage in dogs (Richardson, 2000). Doses within certain ranges have different clinical signs, such as 50–125 mg/kg causing vomiting diarrhoea, nausea, anorexia, gastric ulceration and abdominal pain (Richardson, 2000). Doses above 175 mg/kg have the previous signs as well as renal failure (Richardson, 2000). At or above 400 mg/kg results in central nervous system (CNS) effects such as seizure, ataxia, and coma (Richardson, 2000).
Diagnostic tests
Initial blood biochemistry was performed by the inhouse laboratory. Results showed values within normal limits (WNL). Blood biochemistry continued to be analysed on a daily basis. Specific tests ran were creatinine, blood urea nitrogen (BUN), potassium, sodium, and packed cell volume/total plasma protein (PCV/TPP). On day 2 urine was collected via cystocentesis, and a full urinalysis was performed with no sediment or casts noted, urine specific gravity (USG) was 1.012 with small amount of protein present with no other abnormalities.
When compared with fluid rates being administered to the patient, results of the daily blood tests revealed that blood creatinine increased dramatically after fluid rates were dropped. As creatinine is a renal indicator, high levels show a decrease in the function of the kidneys (Bassert and Thomas, 2013). The patient was diagnosed by the veterinarian to be in acute renal failure on day 4 of hospitalisation.
Nursing interventions
Record keeping of the patient's status and treatments is vital in for communication within the practice (Bassert and Thomas, 2013). It increases the patient's level of care and supports the business legally if any complaints are filed (Bassert and Thomas, 2013). Documentation should include any medications, observations, catheter care, treatment plans, identification of staff performing treatments, the time of these treatments, use of any specific equipment which may alter consistency of results, and list consumables used.
Intravenous (IV) catheter maintenance
As this patient required fluid therapy an IV catheter was placed and maintained for the entirety of hospitalisation. One nursing intervention that can be implemented by the veterinary technician/veterinary nurse (VT/VN) in this case is IV catheter maintenance.
Aseptic technique should be followed for catheter placement, including clipping of hair, surgical preparation of skin, and sterilisation of hands (Soifer et al, 1998; Beal and Hughes, 2000; Marsh-Ng et al, 2007; Goddard, 2010). However, studies in human medicine have shown that maintenance of a catheter is more important in preventing infection than insertion technique (Soifer et al, 1998).
A number of steps are involved in maintaining an indwelling IV catheter. Prevention of gross contamination can be achieved by placing a bandage over the catheter site, this also aids in the stabilisation of the catheter to the leg (Goddard, 2010). If bandages become wet, soiled or displaced they should be replaced (Mathews et al, 1996).
Inspection of the catheter insertion site should be conducted at least once daily (Goddard, 2010). This check involves unwrapping the bandaging from around the leg, inspecting for signs of complications including phlebitis or infection, and ensuring the patency of the catheter (Goddard, 2010; Soifer et al, 1998). This check should coincide with regular flushing of the catheter itself. In patients without continuous IV fluids, flushing should be performed every 4 hours with either saline or heparinised saline (Mathews et al, 1996). When performing a flush, there should also be an inspection of the leg and surrounding area for subcutaneous pockets of fluids (Mathews, 2012). If resistance is felt when flushing, a full examination of the catheter and surrounding site should be performed to ensure that the catheter remains inside the vein (Macklin, 2010). It should also be noted that it can be useful to reposition the animal's leg as the catheter may be occluded (Macklin, 2010). Before every administration of flush or IV medications, the ports of the catheter or fluid line should be swabbed with an antiseptic to ensure that no microbes are introduced (Tan et al, 2003).
Research into IV catheters in animals has shown the dwelling time to be unrelated to infection rates (Mathews et al, 1996). This means that an adequately maintained IV catheter can remain in place until the end of hospitalisation, as long as there are no complications (Mathews et al, 1996). Table 1 summarises the IV catheter care.
| Aseptic placement |
| Place ointment and sterile dressing over catheter site |
| Placing bandage around catheter and leg |
| Inspection at least once daily |
| Flushing every 4 hours |
| Swab ports before injection anything |
| Catheter removed if complications are noted |
Monitoring IV fluid administration for overload
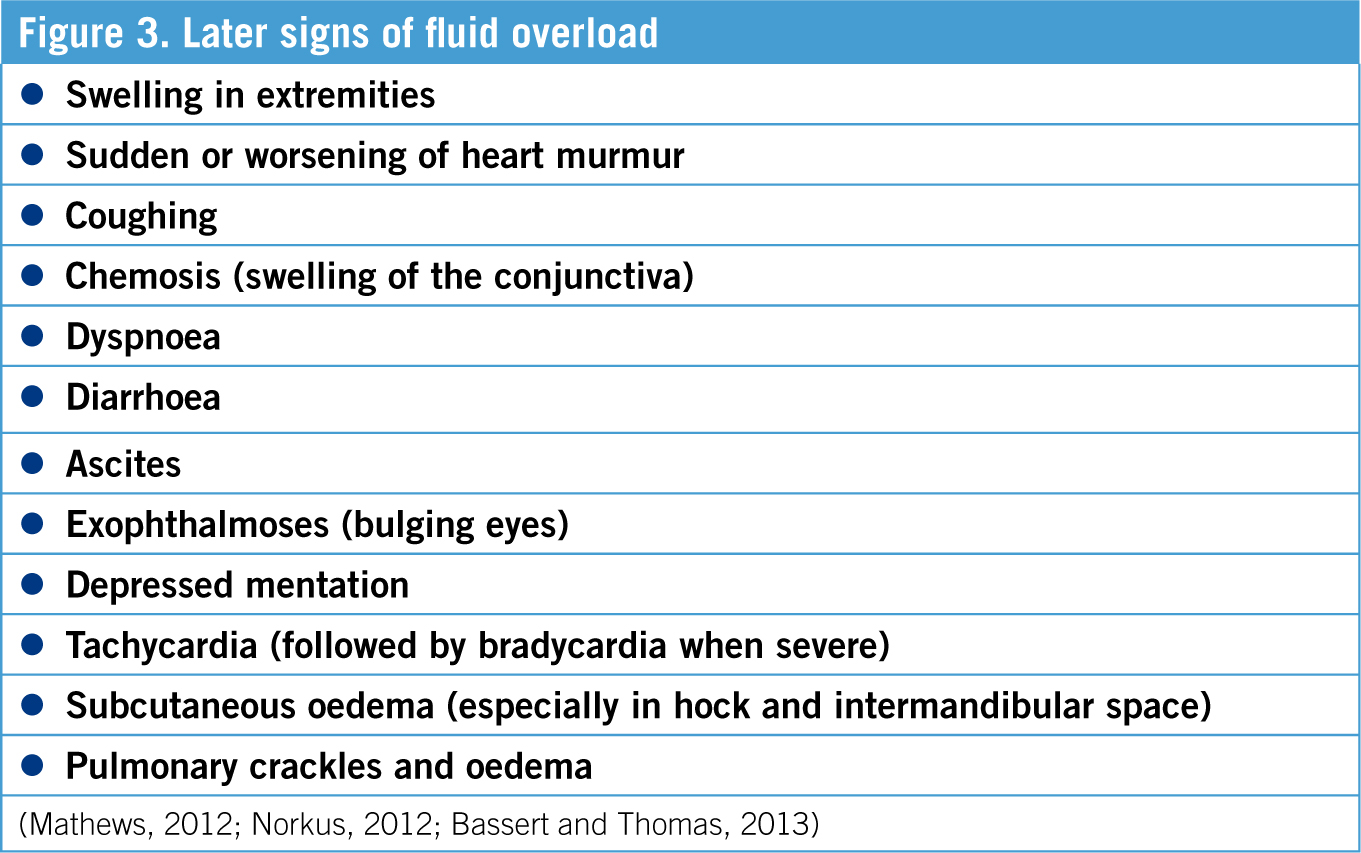
Another nursing intervention that can be performed by the VT/VN in this case is monitoring of fluid therapy administration. Patients on fluid therapy should be monitored to ensure the fluids are being administered as per instructions, and they also need to be watched for signs of fluid overload. A VT/VN who is educated on signs of fluid overload is instrumental to early detection (Norkus, 2012).
During fluid therapy the aim is for mean arterial blood pressure (MAP) of 70–90 mmHg, overshooting this can lead to negative effects (Norkus, 2012). Therefore the gold standard of monitoring is by constant or regular blood pressure readings. However fluid overload is also detectable by other means, including a simple temperature, pulse and respiration check (TPR) (See Tables 2 and 3). An increase in respiratory rate is often the first sign of fluid overload, therefore monitoring of the respiratory rate is essential (Norkus, 2012). If a respiratory rate increase of 20% or more with an increased respiratory effort and pulmonary crackles is observed, fluid administration should be stopped and brought to the attention of the veterinarian (Bassert and Thomas, 2013). The major concern of fluid overload is the development of pulmonary oedema, which is detected as crackles on auscultation of the chest (Bassert and Thomas, 2013).
| Shivering |
| Nausea (swallowing and licking lips) |
| Vomiting |
| Restlessness |
| Polyuria (patient dependent) (>2 ml/kg/hour) |
| Serous nasal discharge |
| Increase bodyweight |
| Tachypnoea |
| Increased respiratory effort |
Tachycardia can be a response to both decreased blood volume and fluid overload, therefore other factors have to be assessed in order to determine whether fluid overload is the cause (Bassert and Thomas, 2013). The pulse should be strong and synchronous in times of fluid overload and healthy status (Bassert and Thomas, 2013).
Patient hydration status is best monitored by frequent weighing and tracking of the bodyweight, preferably twice daily (Bassert and Thomas 2013). A change of weight by 1 kg is 1 litre of body water (Bassert and Thomas, 2013). Monitoring urine output (UOP) is also a good indication of hydration, with a normal UOP being 1–2 ml/kg/hour (Mathews, 2012). Measuring PCV/TP is also useful to monitor the patient's response to fluid therapy (Chapman, 2012).
Urinary catheter maintenance
Due to the potential renal toxicity of ibuprofen, the patient's UOP needed to be monitored as UOP is an important part of monitoring kidney function and hydration status (Oosthiuzen, 2011). The placement of an indwelling urinary catheter enables UOP to be monitored accurately (Li et al, 2013). The maintenance of the indwelling urinary catheter is another nursing intervention which can be performed by the VT/VN. In order to monitor UOP, the collection bag should be measured every 4 hours using an aseptic technique, and UOP calculated (Oosthiuzen 2011). The following equation can be used to calculate UOP:
ml of urine collected ÷ hours since last emptied ÷ patient weight = UOP ml/kg/hour
Complications caused by indwelling urinary catheters can include urinary tract infection (UTI), cystitis, urethral or bladder trauma, blockage or obstruction, and self mutilation or removal (Oosthiuzen, 2011). The longer an indwelling urinary catheter remains in place, the more likely an animal is to develop a UTI, therefore urinary catheters should be removed as soon as they are no longer required (Li et al, 2013). The risk of UTI is increased because placement of a urinary catheter eliminates the patient's natural defence mechanisms which clear bacteria and prevent UTI (Segev et al, 2013). Studies have shown that the incidence of UTI increases by 27% each day an indwelling urinary catheter remains in place (Segev et al, 2013). Signs of UTI can include pyrexia, dysuria and change in colour of urine (Li et al, 2013). See Table 4 for the required maintenance of indwelling urinary catheters.
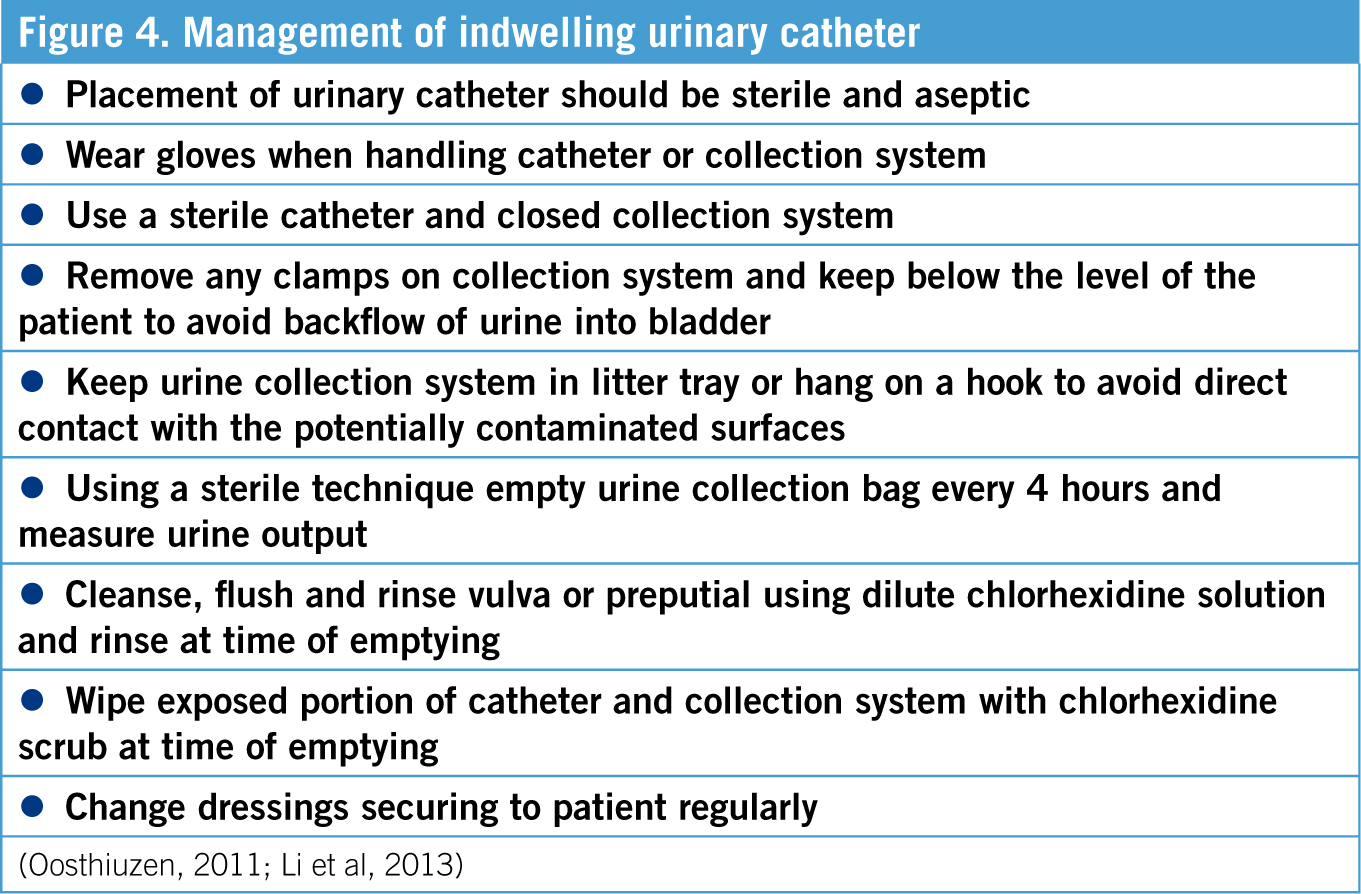
A closed collection system is a urinary catheter attached to a collection bag, and is therefore not open to outside air (Oosthiuzen, 2011; Bloor, 2013). Sterile urinary collection bags are available, however empty fluid bags can be used as urine collection bags if stored appropriately (Bloor, 2013). Appropriate storage of fluid bags involves leaving the existing spike from the original giving set in place, clamping off the tubing and tying a knot to prevent bacteria ascending into the bag (Bloor, 2013). When the bag is needed for urine collection the remaining portion of the giving set can then be removed and the new sterile giving set attached (Bloor, 2013).
Frequent flushing of the urinary catheter can increase the risk of infection due to exposure to the external environment, therefore flushing should be minimised (Oosthiuzen, 2011). If the patency of the urinary catheter is questionable, sterile saline can be flushed through the catheter into the bladder using the most proximal port (Oosthiuzen, 2011). The amount of any saline flushed into the system should be noted or withdrawn from the system so that subsequent UOP calculations remain accurate (Oosthiuzen, 2011).
Patient outcome
The patient was able to go home after an extended stay in hospital. His kidneys recovered enough to have kidney indicators within the normal range and he was booked in for follow ups at his usual veterinary hospital. Follow ups were recommended to be weekly then booked in based on the judgement of the regular veterinary surgeon. The outcome of these follow ups were not available.
Conclusion
The patient was hospitalised for 8 days, during which IV catheterisation, urinary catheterisation and fluid therapy were required. Nursing interventions such as IV and urinary catheter care and fluid monitoring can help to reduce complications caused by hospitalisation. With the correct knowledge and training, VT/VNs can play a vital role in ensuring the correct protocols are implemented in these nursing interventions. Currently these nursing protocols are considered gold standard in veterinary nursing. Every clinic should have some system in place to monitor every patient closely for particular complications that can arise due to treatment. These are just a few, to help lift the level of care given to every animal within a VT/VN's care.

