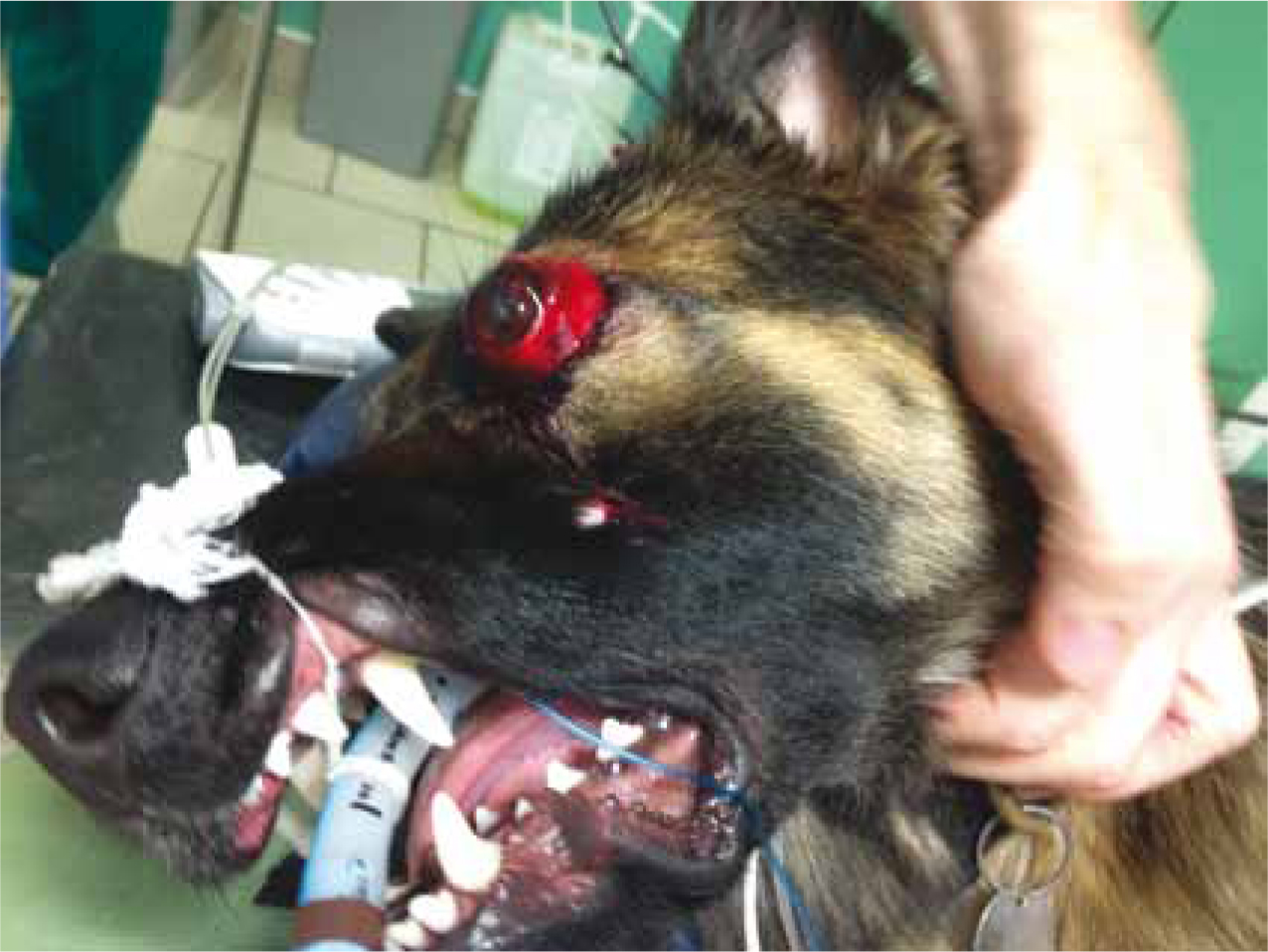
When head trauma cases are considered in veterinary medicine there is a tendency to think of the trauma cases such as a dog kicked in the head by a horse or a cat that has fallen from a high rise building that may potentially be referred elsewhere. It is however important to remember major trauma cases with potential traumatic brain injury (TBI) are seen routinely within general practices in the form of the many cats and dogs that get hit by motor vehicles. Careful consideration of the neurological state of these poly-trauma patients (Figures 1 and 2) should be made alongside cardio-vascular, respiratory and other assessments before considering and planning an anaesthetic.
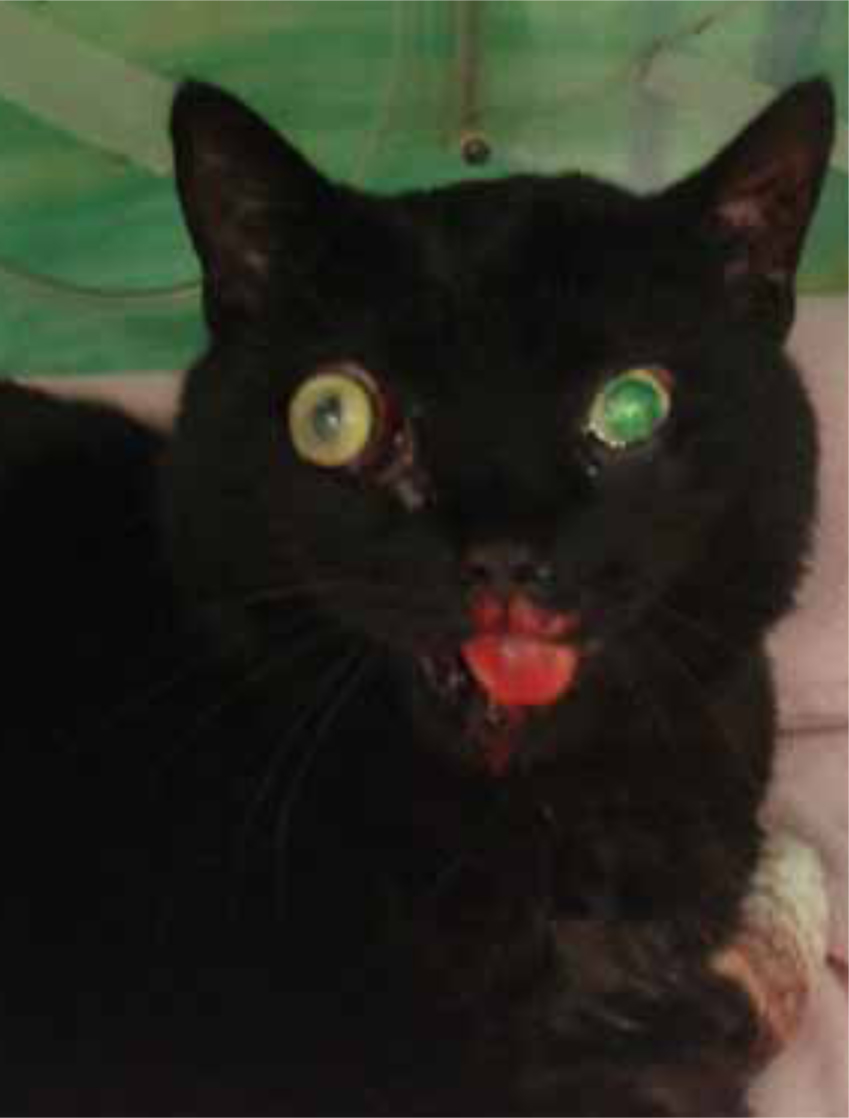
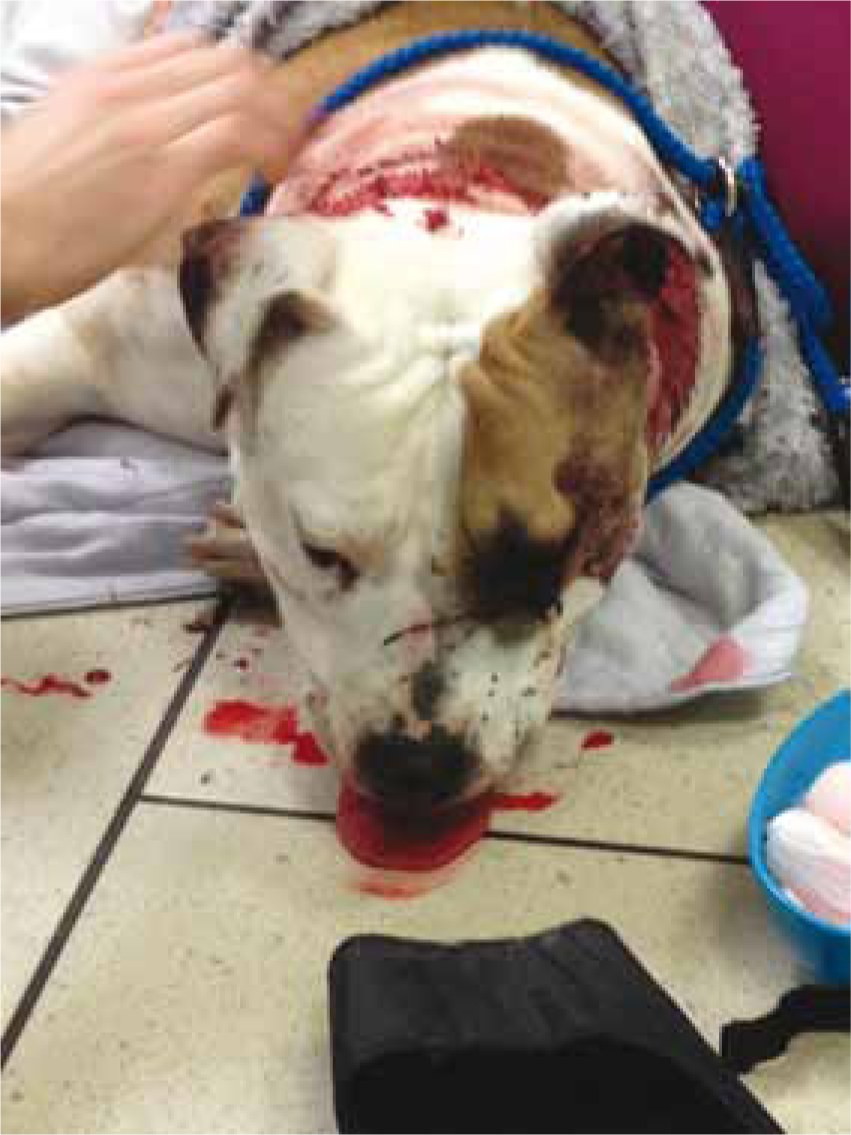
There are many differing reasons for anaesthetising a head trauma patient and these include: diagnostic imaging; surgery; and in severe cases, to control ventilation. It is important for both veterinary surgeons and veterinary nurses alike to recognise the signs of head trauma and consequent TBI and understand the physiology of the ‘normal’ and injured brain to enable all concerned to formulate an appropriate anaesthetic plan and critically evaluate the potential adverse events and how these will be managed. The first consideration for the veterinary surgeon should be whether or not anaesthesia is necessary at the present time or whether it can wait for the patient to be stabilised. This could be either short-term or long-term stabilisation and may require delaying anaesthesia for a few minutes, hours or even days.
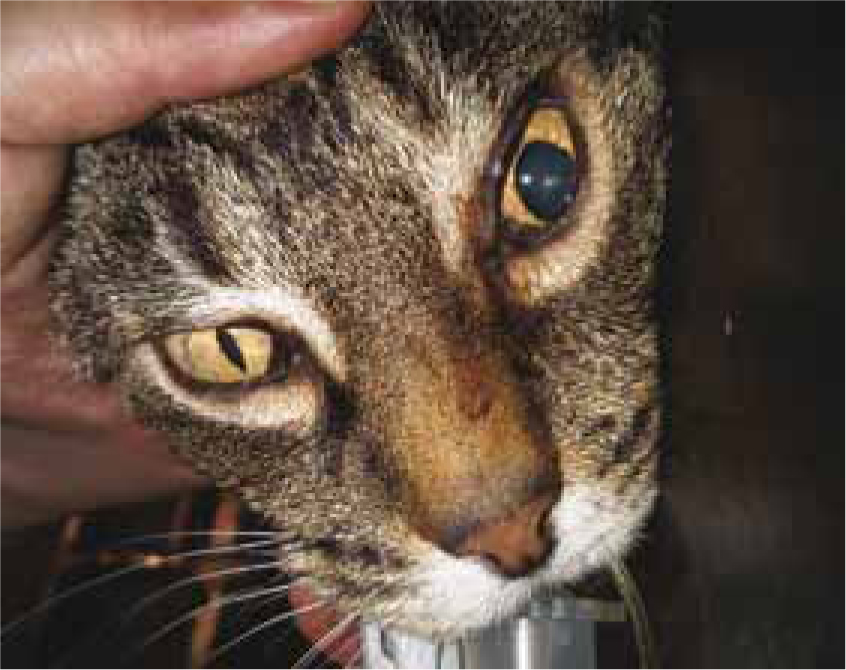
As with all anaesthesia, thorough pre-anaesthetic assessment is vital. This should include: full clinical examination by a veterinary surgeon; evaluation of the patient's clinical history; and stabilisation where necessary prior to induction of anaesthesia. Neurological examination alongside the thorough routine clinical examination is vital in these cases and may reveal signs of intracranial pressure (ICP) such as: poor mentation (sometimes unconsciousness); cranial reflex deficits; head pressing; seizures; disorientation; circling; circulatory and respiratory abnormalities; and uneven pupil size (Figure 3). Neurological examination in human head trauma patients is performed rapidly using the Glasgow Coma Scale (GCS). This can also be performed in dogs using the Modified Glasgow Coma Scale as an assessment of neurological status and a predictor of outcome (Platt et al, 2001). Other baseline parameters that the veterinary surgeon may require to aid patient assessment may include: haematology; biochemistry; blood glucose; electrolyte status; packed cell volume; haemoglobin level; total protein; and urine specific gravity. This will of course depend on the severity of the head injury, the extent to which raised ICP is suspected, whether cerebral haemorrhage is suspected and whether diuretic drugs are indicated to counteract cerebral oedema.
Optimal management of patients with significant TBI must: support oxygen delivery to the brain by maintaining cerebral perfusion pressure (CPP), autoregulation, flow-metabolism coupling and carbon dioxide reactivity; treat initial intracranial hypertension and prevent subsequent increases in ICP; and should evaluate the effects of anaesthetic agents on cerebral perfusion (Armitage-Chan et al, 2006). Analgesia is necessary to maintain animal welfare and limit the adverse physiological consequences of pain and secondary conditions such as hypoxaemia, hyper- and hypo-carbia, and hypo and hyper-glycaemia must be avoided (Sharma and Vavilala, 2012). Essentially the aim is to keep the physiology of the potentially injured brain as ‘normal’ as possible while endeavouring to provide balanced anaesthesia and analgesia. This should allow accurate diagnosis and/or treatment of patients while maximising safety and comfort.
Brain function depends on maintaining the cerebral circulation to ensure delivery of oxygen and glucose to the brain. This occurs within the relative confines of the cranium which leaves very little space for any increase in the size of the brain resulting in a raise in ICP. TBI is caused by primary mechanical impact which may result in the following: skull fracture; brain contusions; vascular and parenchymal injury and consequently intracranial bleeding; and cerebral oedema (Sharma and Vavilala, 2012). The initial period and subsequent days following TBI can be critical as secondary deterioration can occur following ongoing haemorrhage and cerebral oedema leading to further elevations of ICP and reductions in CPP.
ICP is normally 7–12 mmHg in dogs and cats (Armitage-Chan et al, 2006). However, there is no easy way of measuring this and invasive measurements of ICP, while standard in human neuro intensive care, are not routinely carried out in even the most highly technical referral centres. Diagnosis of ICP is therefore based on clinical findings although magnetic resonance imaging may be suggestive of signs indicating raised ICP such as oedema.
CPP is determined as the difference between mean arterial blood pressure (MAP) and the ICP:
CPP = MAP – ICP
The perfusion of the brain is largely dependent on the CPP and subsequently on the difference between the patient's MAP and ICP (see equation above). As MAP increases or ICP decreases the CPP will increase and as MAP falls or ICP increases the CPP will fall. As a consequence, particular attention should be paid to blood pressure monitoring in these patients with MAP ideally kept at 60–80 mmHg (Armitage-Chan et al, 2006). Where there is a known increase in ICP improved brain oxygenation has been demonstrated with a MAP above 90 mmHg (Armitage-Chan et al, 2006). The veterinary surgeon will advise on prescriptive fluid therapy rates and possibly drug therapy to maintain blood pressure.
In turn, cerebral blood flow (CBF) is determined by CPP divided by the cerebral vascular resistance (CVR):
CBF = CPP/CVR
It should be noted that the higher the ICP becomes then the higher the MAP will need to be to compensate and maintain CBF. If CPP drops to a level where CBF cannot be maintained then the brain does not receive sufficient oxygen and glucose for cellular metabolism to continue with resultant brain damage and potentially death occurring.
Autoregulation is a compensatory mechanism which constricts or dilates the cerebral blood vessels in order to maintain blood flow at a constant level over a wide range of perfusion pressures, typically 50–150 mmHg (Armitage-Chan et al, 2006). This functions in the ‘normal’ brain, with CBF being maintained by alterations in vasomotor tone (vasoconstriction and vasodilation). This is in turn regulated by changes in partial pressure of oxygen and carbon dioxide in arterial blood and by MAP (Armitage-Chan et al, 2006). However in the injured brain these mechanisms may be lost and the CBF becomes more dependent on MAP as described above. CVR will be more influenced by changes in the partial pressure of primarily carbon dioxide. When carbon dioxide levels increase, a corresponding decrease in pH occurs which results in vasodilation in a linear fashion. This reduces CVR, increases CBF and subsequently increases ICP. Hypocapnia results in an increase in pH, vasoconstriction, increased CVR and a decrease in CBF and ICP. Mechanical ventilation therefore becomes a useful tool for manipulating ICP. Recent human recommendations advise avoiding prophylactic hyperventilation (<25 mmHg) but advocate it as a temporary measure to reduce known elevations in ICP (Sharma and Vavilala, 2012). Veterinary recommendation indicates ventilation to eucapnia, i.e. 40 mmHg to prevent ischaemic damage associated with mild hypocapnia (30–35 mmHg) (Figure 4) (Armitage-Chan et al, 2006). Hypoxaemia (i.e. low arterial partial pressure of oxygen) causes vasodilation of cerebral vasculature, increases CBF and can cause an increase in ICP if left uncorrected.
Cushing's reflex (Cushing's triad) can occur in patients with severely raised ICP. Where MAP becomes lower than ICP, an ischaemic response is initiated by the hypothalamus resulting in a massive discharge of the sympathetic nervous system (Cushing's reflex). This causes peripheral vasoconstriction resulting in hypertension, aimed at restoring CBF, alongside a reflex bradycardia. Where respiratory disturbances are also noted due to decreased brain stem perfusion or compression of the respiratory centre then Cushing's triad is complete. This finding indicates a dramatic deterioration and is immediately life threatening.
Nursing interventions and monitoring
There are some simple nursing strategies and anaesthetic considerations that should be employed in all suspected head trauma patients to reduce the risk of increasing ICP further:
Drug considerations for the head trauma patient
Ultimately the choice of anaesthetic and analgesic agents utilised for any head trauma case is down to the risk–benefit analysis of a veterinary surgeon. It is important however for the veterinary nurse to understand how the various agents can affect neurological function, and in particular CBF, cerebral metabolic rate of oxygen (CMRO2) and ICP, to allow them to appropriately monitor the patient in the peri-anaesthetic period.
Premedication
The premedication agents chosen will depend on the individual's neurological state. Generally these cases are exhibiting signs of depression and analgesia is the only form of premedication necessary. The opioids exert minimal effects on CBF and ICP and at clinical doses significant respiratory depression is not often encountered, although it should be noted that side effects will be exacerbated in patients with raised ICP (Armitage-Chan et al, 2006). Morphine is not licensed for use in dogs and cats and may cause vomiting as a side effect which in turn may raise ICP and therefore should be avoided. Methadone and buprenorphine are more suitable choices. Where more profound sedation is required alongside analgesia, medetomidine and dexmedetomidine have been effectively utilised in some settings. These agents appear not to influence ICP in dogs but reduction in heart rate and cardiac output may have an effect on CPP so very low doses are advised when these drugs are used (Armitage-Chan et al, 2006). There is generally little evidence of the effects of these drugs on the cerebral vasculature in veterinary patients. It should also be noted that these drugs may also induce vomiting and should be used with caution.
Induction agents
Induction agents traditionally used in human medicine for the induction of anaesthesia in TBI patients include: thiopentone, propofol and etomidate all of which cause cerebral vasoconstriction, reduce CBF, CMRO2 and ICP (Sharma and Vavilala, 2012). However, propofol and thiopentone may cause cardiovascular depression, which may lead to hypotension, and etomidate offers cardiovascular stability, but may impair adrenal function and lead to delayed hypotension (Sharma and Vavilala, 2012). A recent review also concluded that there was no evidence to suggest that propofol offered perioperative brain neuroprotection, a property widely reported elsewhere (Bilotta et al, 2013). No real consensus as to the best agent to use exists. In veterinary medicine out of the three agents available in human medicine only propofol is appropriately licensed for dogs and cats. Alfaxalone (Alfaxan®, Jurox UK Ltd) is also available and licensed in dogs and cats in the UK. Alfaxalone is not contraindicated in acute head injury but nor are there any studies comparing its use for this purpose (in the veterinary formulation) with propofol. This is highly likely to be because the product in not available for human patients at this time. Previous studies of alfaxalone in combination with alphadalone (Althesin) indicate decreased CMRO2, CBF and ICP while preserving CPP in people following head trauma (Dearden and McDowall, 1985). One recent study compared the use of alfaxalone in ASA 3–5 patients with a diazepam/fentanyl co-induction and concluded that alfaxalone resulted in similar cardiorespiratory effects and is a clinically acceptable induction agent in sick dogs (Psatha et al, 2011). This study did not specifically look at head trauma but in combination with the previous work on alfaxalone/alphadalone in combination may mean alfaxalone is a potentially beneficial alternative to propofol. It should also be noted that recent reviews of TBI in children recommend that propofol should be avoided due to potential morbidity and this echoes the stance of the FDA (2001) that ‘propofol is not approved in the USA for sedation in paediatric ICU patients’ (Hardcastle et al, 2014). Benzodiazepines may be useful as part of a co-induction protocol for TBI as they decrease CBF and ICP and may reduce the dose of induction agents such as propofol (Armitage-Chan et al, 2006).
Inhalational agents
The inhalational agents isoflurane and sevoflurane both decrease CMRO2 but may cause cerebral vasodilation and therefore raised ICP if administered at a concentration higher that 1 minimum alveolar concentration (MAC) (Sharma and Vavilala, 2012). It is therefore possible to justify the use of the agents but only in low concentrations where TBI is suspected (Sharma and Vavilala, 2012). For this reason some anaesthetists would recommend total intravenous anaesthesia (TIVA) as a preferable alternative to the inhalational agents where raised ICP is suspected and this has traditionally been done using propofol (Armitage-Chan et al, 2006). A risk–benefit analysis of the available drugs for TIVA should be conducted by the veterinary surgeon and the VS should have experience using this technique. Nitrous oxide cannot be recommended for use in patients with TBI as it causes a dramatic increase in CBF and consequently ICP (Sharma and Vavilala, 2012).
Dissociative agents
Ketamine had previously been avoided in cases of TBI as it was thought to increase ICP. It has more recently been investigated with studies showing that in adequately ventilated patients its use demonstrated either no change or a reduction in ICP and electroencephalography (EEG) activity (Chang et al, 2013). There is also possible indication that ketamine may provide neuroprotective benefits in patients at risk of ischaemic brain injury, reducing cell death and neuronal degeneration (Chang et al, 2013). It has also been used in paediatrics to mitigate increases in ICP and to treat refractory intracranial hypertension (Hardcastle et al, 2014). These studies have been conducted in human patients with TBI and the authors of a recent review state that there are encouraging signs that the use of ketamine in these patients may increase in the future but that the data are too sparse to provide firm guidance (Chang et al, 2013). This is certainly an area where future research is warranted in both the human and veterinary sectors.
Hyperosmolar therapy
Hyperosmolar therapy may be indicated to counteract the cerebral oedema in cases where there is a known increase in ICP. This has traditionally been done using the osmotic diuretic mannitol which increases blood volume and decreases blood viscosity resulting in increased oxygen delivery to the brain and subsequent vasoconstriction and a fast decrease in ICP (Armitage-Chan et al, 2006). Due to osmotic diuresis, possible hypovolaemia and hypotension and the requirement for an intact blood–brain barrier (if the blood–brain barrier is not intact mannitol may enter the brain causing fluid to be drawn in and an increase in ICP instead of the intended reduction) the use of mannitol is only recommended where signs of brain herniation are evident or there are progressive neurological signs not attributable to external causes (Sharma and Vavilala, 2012). Hypertonic saline 7.2% may also be used as an alternative to mannitol given at 4 ml/kg slowly intravenously to decrease ICP and cerebral oedema (Armitage-Chan et al, 2006).
Corticosteroids are contraindicated in patients with moderate and severe TBI as administration has been associated with increased mortality in these cases (Sharma and Vavilala, 2012).
Where vasopressors such as noradrenaline, phenylephrine or dopamine are indicated to increase MAP and CPP it should be noted that there is no current evidence to support the use of one of these drugs over another at the present time (Sharma and Vavilala, 2012).
Fluid therapy is almost always necessary in TBI patients to maintain adequate CPP and this should constitute a warm, non-glucose containing, isotonic crystalloid solution (Sharma and Vavilala, 2012). Hyperglycaemia following the administration of glucose containing fluids is associated with cerebral lactate production and has been associated with poor neurological outcomes (Armitage-Chan et al, 2006). Fluid administration should be monitored carefully to avoid over infusion and prevent increases in ICP and 5 ml/kg/hour is a suggested maximum during anaesthesia (Armitage-Chan et al, 2006).
The recovery of the head trauma patient is much the same as that of any other critical patient and should constitute careful monitoring of all vital parameters alongside mentation and neurological function. Temperature is a consideration and while some human literature has evaluated moderate hypothermia in TBI patients this cannot be recommended and shivering should be avoided due to an increase in metabolic oxygen demand (Armitage-Chan et al, 2006). Hyperthermia should be avoided and patients kept as normothermic as possible. Oxygen therapy should be available and utilised where needed.
Conclusion
In conclusion, it is likely that most general veterinary practices see dogs and cats where there may be the potential for TBI on a semi-regular basis (Figure 5). This article has discussed the physiology of both the ‘normal’ and ‘injured’ brain and it is important to have a clear understanding of what ‘might’ be happening inside that patient's cranium to allow the veterinary nurse to fully identify the potential adverse events and complications that may be encountered. Anaesthetic considerations and simple nursing strategies have been suggested to help formulate comprehensive anaesthetic and nursing plans for patients with suspected head trauma and to prevent the worsening of ICP. While it will always be down to the veterinary surgeon to decide the drug protocols for cases, it is especially important to understand how any drugs administered might affect brain physiology in the TBI case. This will give an understanding of the potential adverse events and facilitate thorough planning and preparation to help prevent them. The nursing component of monitoring and assisting with veterinary support of cardiovascular and respiratory function is of primary importance over drug factors in these high intensity nursing cases.