The patient was found by a member of public and taken to Lower Moss Wood Wildlife Hospital before presenting to the veterinary practice several days later. The patient was anorexic, in poor body condition and unable to fly.
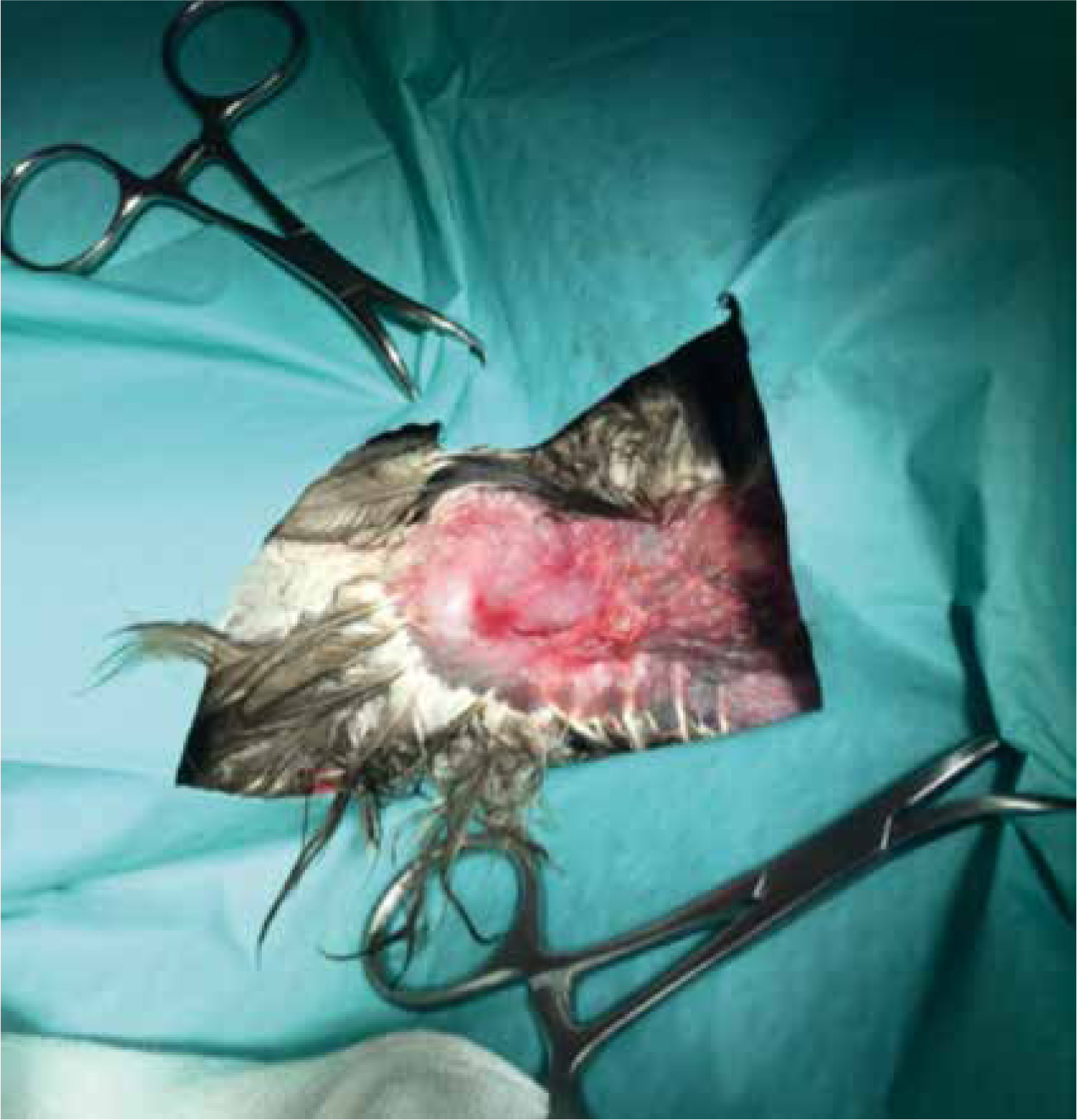
Radiographs revealed an open transverse fracture of the distal humerus. The humerus is a pneumatic bone; the clavicular air sac extends into the medulla. Coles (2007) asserts this to be clinically insignificant as the air sac quickly seals with a blood clot following injury preventing emphysema of the surrounding tissues. It is vital to achieve accurate alignment of the fragments as most of the mechanical forces imposed on the wing during flight are transmitted to the humerus; however, in some cases birds can fly despite adequate alignment (Coles, 2007).
The Animal Welfare Act 2006 states that any wild animal brought into a veterinary practice should receive appropriate treatment to relieve pain and suffering, this includes surgery but also euthanasia. Under the Abandonment of Animals Act 1960 it is an offence to release into the wild an animal that is not fit to survive or that is suffering (Fraser and Girling, 2016). The veterinary surgeon (VS) involved considered that the fracture was repairable and that following rehabilitation the patient would be able to fly and subsequently be released. If during surgery the VS had decided that this was not the case, the patient would have been euthanased on welfare grounds.
Signalment
Preparation for anaesthesia
The patient was transported to the practice in a wire cage. To minimise stress the patient was kept in this cage following initial clinical examination. The patient was kept in a warm, quiet room, away from predators, e.g. cats and dogs, with dim blue lighting. Dim blue or red lighting is recommended as it prevents the bird from seeing the handler, which minimises stress. Where possible birds should be housed in separate wards (exotics or birds only). Ideally birds should have their own air space and ventilation system (air extracted from individual kennels) to minimise the spread of disease via the respiratory route (Abou-Zahr and Forbes, 2015).
Birds of prey should be hospitalised in a quiet area, with an ambient temperature of 26–30°C and a humidity of 70% (Mancinelli and Bament, 2014). Further recommendations suggest 26.7–29.4°C and advise housing birds in cages that have the ability to be heated (Abou-Zahr and Forbes, 2015). It is also suggested that a perch is provided (Abou-Zahr and Forbes, 2015).
Once the equipment was prepared the patient was captured and restrained gently with a towel. When capturing and restraining birds it is important to use a calm approach to minimise stress (Raftery, 2013). Stress in combination with the use of anaesthetic agents can predispose the patient to cardiac arrhythmias and subsequent catecholamine release, which increases the risk of cardiac arrest and subsequent death.
It is recommended that birds of prey are fasted for 12 hours prior to induction of anaesthesia to ensure the crop is empty and/or a cast has been produced (Mancinelli and Bament, 2014). The crop could be emptied if required (Raftery, 2013). In this case, the patient was anorexic therefore fasting was not required and the crop was empty on admission to the hospital.
Raftery (2013) recommends stabilising patients prior to anaesthesia; as this was a wildlife case, initial stabilisation was limited by funds.
Pre-oxygenation
Prior to induction of anaesthesia the patient was placed in an oxygen chamber where it received supplemental oxygen — 3 litres/minute for 5 minutes. Pre-oxygenation is important as it produces an oxygen reservoir within the air sacs which is four to five times greater than the normal level (Hawkins, 2013). In healthy birds the benefits are achieved in under 1 minute, but in debilitated birds or those with a compromised respiratory function it can take over 5 minutes (Hawkins, 2013)
Induction
Anaesthesia is most commonly induced using either an anaesthetic chamber or face mask. Raftery (2013) recommends a clear face mask with a flexible non-porous diaphragm. Patients can also be induced using combinations of injectable drugs such as butorphanol (1 mg/kg) with ketamine and medetomidine or midazolam (Coles, 2007). There are variable effects of injectable drugs associated with poor induction: inadequate muscle relaxation, cardiopulmonary depression and prolonged or violent recoveries (Hawkins, 2013). In comparison, induction with inhalational agents has been shown to be rapid, with rapid, smooth recoveries (Hawkins, 2013).
In this case, the patient was induced in an oxygen chamber using 2.5–3% isoflurane and an oxygen flow rate of 3 litres/minute. Coles (2007) and Raferty (2013) recommend 5% isoflurane and 2 litres/minute oxygen and 4–5% isoflurane and 1 litre/minute oxygen respectively, but as the patient was debilitated lower levels of isoflurane were sufficient in this case. Care should be taken if inducing anaesthesia in a chamber as monitoring can be difficult. Raftery (2013) states sedation is achieved in less than a minute followed by a surgical plane of anaesthesia in 2–3 minutes (Coles, 2007).
Intubation
Raftery (2013) recommends intubation of all birds over 200 g. In this case the patient was intubated using the following technique.

Care should be taken not to insert the ETT too far as the trachea in birds narrows distally (Girling, 2008; Raftery, 2013). Damage to the tracheal lining and subsequent stenosis can occur easily but is often not apparent for 2 to 3 months. The risk of damage can be minimised by lubricating the ETT, choosing an ETT of the correct size without a cuff and keeping the trachea straight during surgery (this also prevents kinking of the ETT). Unlike in canine and feline patients where a snug fit is recommended, in avian patients the ETT should loosely fit in the glottis, this allows excess gas to escape thus preventing over inflation of the air sacs or damage to the trachea. The length should also be cut short to minimise dead space. For small patients a canine urinary catheter can be adapted for use as an ETT by cutting off the end and attaching to an ETT adapter (Coles, 2007).
The ETT should be connected to a suitable anaesthetic circuit, in this case a modified Ayre's T-piece was used. A non-rebreathing semi-open circuit such as a modified Ayre's T-piece or mini Bain coaxial circuit should be used as many birds do not have sufficient tidal volumes to move the anaesthetic gases sufficiently, therefore rebreathing of carbon dioxide can occur. In some cases the patient may require intermittent positive pressure ventilation (IPPV, therefore it should be ensured the circuit used is suitable for IPPV; both the modified Ayre's T-piece and mini Bain coaxial circuit would be suitable. When performing IPPV Coles (2007) recommends use of an open ended rebreathing bag. Hawkins (2013) states a rate of 6–10 breaths per minute (bpm) should be employed. The mini Bain coaxial circuit is often preferred as it warms the anaesthetic gases, minimising heat loss for the patient. Alternatively devices such as thermovents should be utilised to warm the anaesthetic gases. Where possible a humidifier should also be used as high levels of water loss can occur in the air sacs (Coles, 2007; Girling, 2008).
Air sac cannulation can be performed as an alternative to intubation, this was not performed in this case due to limited funds. Air sac cannulas are routinely used for oxygenation and anaesthesia (Brown and Pilny, 2007). Specific indications for air sac cannulation include; head or tracheal surgery where the ETT would be in the VS' surgical field, where there is trauma, collapse or obstruction of the trachea or for dyspnoeic birds such as those with upper respiratory tract foreign bodies or tracheosyringeal obstruction from granulomas or tumours (Brown and Pilny, 2007). The technique used to place an air sac cannula is beyond the scope of this article.
Once anaesthetised the patient's tail feathers were protected using a peel and seal autoclave bag. Mancinelli and Bament (2014) advocate protecting the tail feathers using radiographic film or cardboard as damage during hospitalisation or anaesthesia can affect the bird's ability to fly until the feathers next moult. Care should be taken to ensure the method of protection does not obstruct the cloaca; this is of particular importance in hospitalised patients.
Maintenance
During surgery the patient was maintained on 1–1.5% isoflurane. Coles (2007) and Raftery (2013) state 1.5–3% and 2–4% isoflurane respectively is usually used for maintenance; given this patient's poor condition, lower levels were sufficient. Depth of anaesthesia was gauged by eye position, relaxation of the limbs and neck and a lack of pedal reflex. These are factors Raftery (2013) advises should be monitored (see monitoring section below).
Isoflurane is described by Coles (2007) as the safest anaesthetic agent for avian patients for several reasons:
Positioning
In this case the patient was positioned in sternal recumbancy to facilitate surgery on the left humerus. Dorsal recumbancy is commonly used, however the viscera push anteriorly which can compromise respiration due to compression of the abdominal and thoracic air sacs (Raftery, 2013). Raftery (2013) advocates lateral recumbancy (providing there is no unilateral lung pathology) as this allows for normal respiration.
Consideration should be given to how the patient is draped for surgery and the positioning of monitoring aids. It is vital the VN's view of the patient is not obstructed (Lennox, 2014). In this case, minimal draping was used.
Blood sampling and intravenous/intraosseous catheterisation
Blood sampling was not performed in this patient because of lack of funding. Some birds may require anaesthesia for blood sampling using isoflurane (Longley, 2009). Small gauge needles should be used to prevent damage to the vessels and gentle pressure should be applied following removal of the needle to minimise haemorrhage. 10% of a patient's blood volume can be safely sampled, in birds this equates to 1% of their bodyweight. In ill patients smaller volumes should be taken (Longley, 2009). There are a number of sites that can be used including: the right jugular, basilic/ulna vein and the metatarsal veins (both of which are suitable for catheter placement) (Pollock, 2010).
A number of people recommend placing an intravenous catheter in all birds requiring an anaesthetic for longer than 15 minutes (Coles, 2007; Abou-Zahr and Forbes, 2015). The right jugular can be used in most species and the ulna/basilic veins in larger birds. Haematoma formation is common in birds and significant bleeding can occur if the catheter is dislodged (Pollock, 2010). Intravenous catheter placement was discussed in this case, but due to lack of funding was not performed.
Intraosseous catheters can also be placed in collapsed or anaesthetised birds, again, this was not performed in this patient. It is important to remember birds of prey have pneumatised bones, e.g. femur and humerus; these should be avoided (Mancinelli and Bament, 2014).
Fluid therapy
The most common route for administering fluids is intravenously. Alternatively, fluids can be given via the intraosseous route in the distal ulna or proximal tibiotarsus (Lennox, 2014). Fluids can also be given subcutaneously, Ford (2007) states this can be used to correct mild dehydration or provide maintenance fluids. Fluids can be administered in the axilla, over the dorsal area between the scapulae or more commonly in the precrural fold in the inguinal area. Care is needed when placing the needle as if too deep the abdominal air sac can be penetrated resulting in fluid moving to the lungs (Ford, 2007).
Lactated ringer's and glucose saline are the most commonly used fluids for avian patients (Fraser, 2008; Lennox, 2014). Hartmann's can be given as a constant rate infusion (CRI) at 10 ml/kg/hour or as a bolus (50–100 ml/kg/day) (Mancinelli and Bament, 2014). Fluids should be warmed to 38–39°C (Lichtenberger, 2007a) and if available hyaluronidase (1:100) can be added to the fluids to improve absorption. Thought should be given to the method in which the fluids are administered; a paediatric giving set, infusion pump, syringe driver or intermittent hand injection can be used if the hourly rate is calculated (Mancinelli and Bament, 2014).
In this case the patient received subcutaneous fluids administered by intermittent hand injection over the dorsal area between the scapulae using Hartmann's which was warmed prior to administration.
Medications
The mechanisms by which pain is transmitted is multifactorial; no one class of drug can be used for all pain (Pollock, 2011). Provision of adequate multi-modal analgesia in birds is imperative as pain assessment is challenging. Many clinical signs can be attributed to pain including behavioural changes, restlessness, a reluctance to move, stand or perch, anorexia, over or under grooming and tachypnoea (Hawkins, 2010). The patient in this case was anorexic and unable to use the affected wing.
Opioids are recommended for moderate to severe pain and non-steroidal anti-inflammatories (NSAIDs) for mild to moderate pain (Pollock, 2011). Hawkins (2007) recommends using opioids for pain associated with fractures/trauma and for surgical pain. The effect of opioids in birds is variable and birds generally do not respond well to mu agonists, e.g. morphine and methadone. Studies in pigeons have demonstrated that the distribution of mu, kappa and delta receptors in the forebrain and midbrain are similar to that of mammals but that the mu and delta receptors are more prominent, therefore opioids that act on these receptors will be more effective (Hawkins, 2007). Butorphanol is a mixed agonist/antagonist with low mu activity and strong kappa activity, it is for this reason butorphanol is commonly used in birds. Jiménez (2008) and Raftery (2013) recommend the use of butorphanol (0.5–2 mg/kg every 2–4 hours) and meloxicam (0.5-1 mg/kg every 12–24 hours). Raptors respond well to both butorphanol and buprenorphine (Pollock, 2007), in some species sedation may be observed. Butorphanol can cause cardiac and respiratory depression (Pollock, 2011). NSAIDs have beneficial effects but can cause gastrointestinal and renal side effects; if the patient is hypotensive or dehydrated the likelihood of these side effects is increased (Pollock, 2007). In this case, the patient was given butorphanol (1 mg/kg) intramuscularly into the pectoral muscle and meloxicam (1 mg/kg) once anaesthetised.
Pollock (2011) and Raftery (2013) discuss the use of local analgesia and incisional blocks using lidocaine (<2 mg/kg topically) with bupivacaine (<2 mg/kg topically). Toxic effects can be observed with the use of these drugs so low doses are recommended, diluting the drug may also be beneficial (Pollock, 2011). This was not utilised in this case.
Provision of post-operative pain relief has been shown to reduce the time to return to normal eating and preening. The incidence of wound breakdown and self-trauma can also be reduced (Girling, 2008), in this case the patient was discharged with meloxicam (1–2 drops/kg bodyweight).
The use of antibiotics should be judicious (Pollock 2007). The patient was given enrofloxacin (Baytril 2.5% solution for injection, Bayer) at the time of surgery and discharged with an oral course this was due to the nature of the fracture (open transverse) and the risk of osteomyelitis which can affect healing (Coles, 2007). VSs should be reasonably sure an infection is present before giving antibiotic therapy (Flammer, 2008).
Monitoring
Anaesthesia of avian patients can change rapidly therefore the patient should be given the VN's undivided attention and be monitored continuously. In this case one VN was responsible for monitoring the anaesthesia and a second assisted the VS with opening equipment and also monitored anaesthesia when available. This method is advocated by Lennox (2014).
During anaesthesia the patient's heart rate, respiratory rate, mucous membrane colour and capillary refill time (via observation of the oral mucosa) were monitored. Depth of anaesthesia was also monitored, Table 1 discusses points to monitor at differing stages of anaesthesia. The pedal reflex produces an unreliable response in birds, but particularly in raptors, so this should be taken into consideration. In birds the eyelids may be closed but the corneal reflex should be present albeit sluggish; if the corneal reflex is absent the patient is too deeply anaesthetised (Coles, 2007).
| Stage | Information |
|---|---|
| 1 | Shallow, erratic respiratory rate, lethargic, drooped eyelids, ruffled feathers |
| 2 | As above, palpebral, corneal, pedal and cere reflexes are present, no voluntary movement |
| 3 (light plane of anaesthesia for minor procedures) | Respiratory rate is regular with an increased depth, corneal and pedal reflexes are slow, palpebral reflex is absent |
| 3 (deeper plane for most surgical procedures) | As above but pedal reflex is absent, corneal reflex is present |
For procedures lasting over 5 minutes blood pressure, capnography, core body temperature, electrocardiography and pulse oximetry should be monitored (Raftery, 2013).
Abou-Zahr and Forbes (2015) state capnography to be the most useful method of monitoring avian patients under anaesthesia. Unfortunately, capnography was not available in this case. End tidal carbon dioxide levels >45 mmHg indicate inadequate respiration. This can occur as a result of the patient being too deeply anaesthetised or the positioning of the patient compromising respiration (Raftery, 2013).
A Doppler probe and stethoscope (placed over the pectoral area) were used to monitor the patient's heart rate. The probe was placed over the superficial ulna/basilic vein on the ventral aspect of the elbow joint and medial tarsal vein on the medial aspect of the lower leg. A carefully placed probe if undisturbed can also enable blood pressure to be monitored, decreasing volume or intensity may indicate decreasing blood pressure (Lennox, 2014).
Raftery (2013) recommends monitoring blood pressure. Both Doppler and oscillometric monitoring can be used, however, Doppler is considered to be more accurate for smaller patients. A cuff (the width should be 30–40% of the limb circumference) should be placed on the distal humerus or femur for birds weighing over 300 g. The Doppler probe is then placed over the proximal or distal ulna or the metatarsal arteries (depending on the chosen site) (Lichtenberger, 2007b). The method for blood pressure monitoring is the same as for canine/feline patients. Studies have suggested the numbers obtained when monitoring blood pressure in avian patients may not be a true reflection of the patient's blood pressure, instead it is recommended that trends are monitored (Johnston et al, 2011).
A pulse oximeter was used to aid monitoring of the patient, the probe was placed in the same location as the Doppler probe (see above); it is recognised that pulse oximetry is of limited benefit in avian patients due to differences in haemoglobin structure from that of mammals. It was however utilised to monitor trends as recommended by Lennox (2014).
Electrocardiography (ECG) was not used in this patient, however, Raftery (2013) states it can be useful to monitor for bradycardia and ST segment depression which are often the first signs observed with anaesthetic overdose. It is important to use an ECG monitor that is calibrated for birds and to remember an ECG may appear normal in cases of severe acute cardiopulmonary compromise (Raftery, 2013).
While in theatre the ambient temperature was maintained at 24°C. Jiménez (2008) recommends an environment of 28–32°C for debilitated birds as in this case. Studies of selected species of birds have demonstrated a reduction in core body temperature 20 minutes following induction of anaesthesia. If this is not rectified quickly cardiovascular instability and poor recovery can occur and there is a decreased chance of patient survival. Minimal plucking of the surgical site was conducted, and care was taken not to soak/excessively wet the area (Figure 2). The patient was supported in theatre using a ‘hot dog’ electric heat pad, other methods of thermal support include forced air warmers, radiant heat and warm water blankets (van Zeeland et al, 2012). Temperature was monitored intermittently via the cloaca using a digital thermometer. It is, however, recommended that a flexible temperature probe is used for continuous monitoring, this can be placed in the cloaca or crop (Lennox, 2014).
Recovery
It is recommended that patients are positioned upright to prevent compromising normal respiratory movement (Coles, 2007; Raftery, 2013; Mancinelli and Bament, 2014); the patient should be held upright using a light grip by a competent VN (Coles, 2007; Raftery, 2013). Wrapping the patient with a towel is also advised as this prevents the patient from flapping its wings and causing damage (Girling, 2008). In this case, the patient was maintained on 100% oxygen via the endotracheal tube, once moving his head the patient was extubated and then held upright secured in a towel (Figure 3).

Recovery from isoflurane alone is usually complete within 5 to 10 minutes, as was the case in this patient. If other agents are used, e.g. ketamine, recovery can take several hours (Girling, 2008).
Monitoring should continue into the recovery period, in this case the patient's respiratory rate and depth were monitored. In addition sternal excursions should be monitored. If recovery is slow, moving the patient's wings in and out can stimulate respiration (Raftery, 2013). This was not required in this case but would also not have been advised given the nature of the surgery. If an intravenous or intraosseous catheter had been placed this would be removed along with any monitoring aids, unless they were required for post-operative care (Raftery, 2013).
Raptors can regurgitate on recovery (Raftery, 2013), this was not seen following initial surgery but was observed when the patient returned for post-operative x-rays 6 weeks later, it is therefore vital to monitor patients closely.
The patient recovered well (Figure 4) from anaesthesia and was later discharged back to the wildlife hospital to receive further rehabilitation and care.

Patient outcome
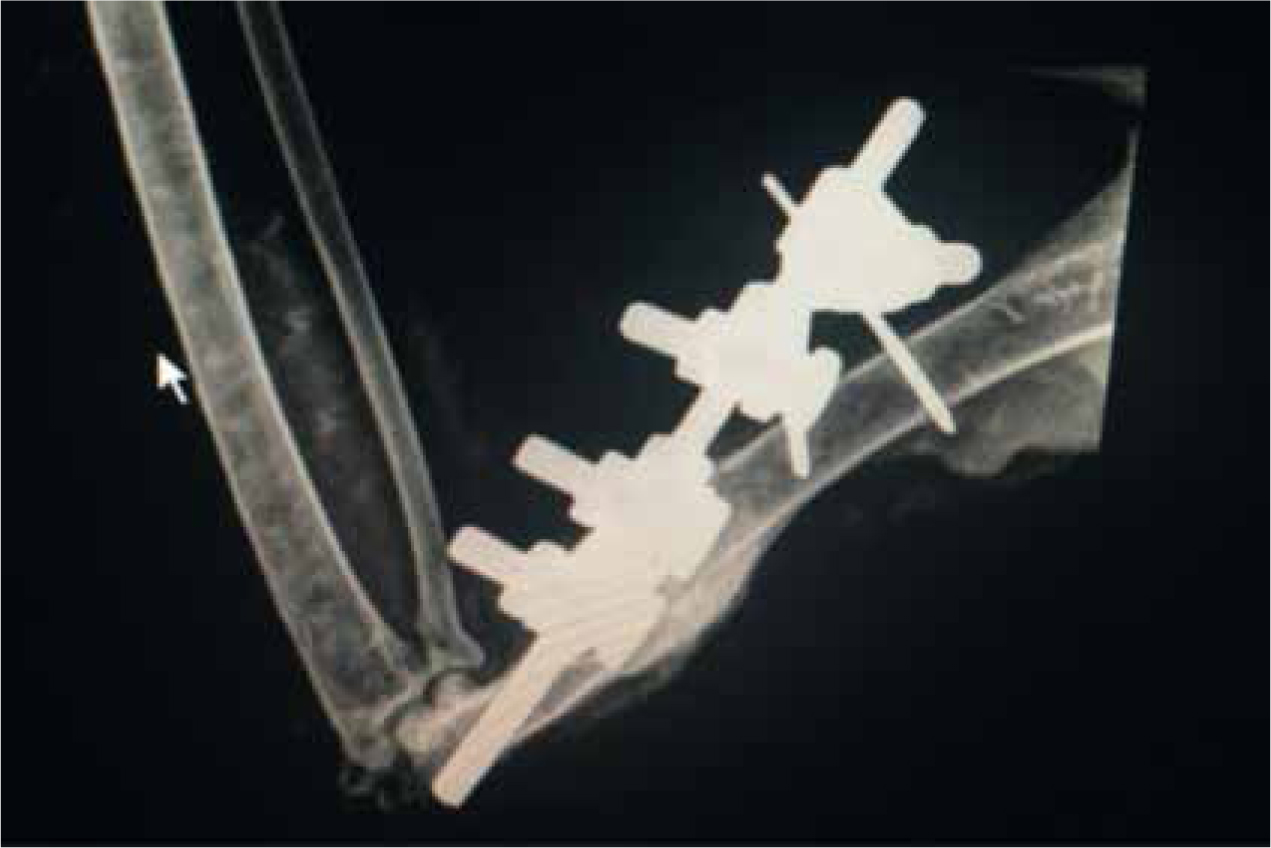
The patient involved had an external fixator placed (Figures 5 and 6) which was removed 6 weeks later following postoperative radiographs. The patient received further rehabilitation and was released back into the local environment.

Conclusion
This case highlights the need for VNs to be passionate and vigilant when monitoring avian patients as changes in anaesthesia can occur rapidly. A good outcome was achieved for this patient despite a lack of funding. In future cases, if funds allow, placement of an intravenous or intraosseous catheter and pre-anaesthetic blood sampling are recommended to allow vascular access and to assess any comorbidities which may affect the treatment plan. Additional monitoring aids such as a capnograph and ECG would have been beneficial to enable closer monitoring. With wildlife cases it is vital the ethical and welfare implications of surgery are considered to ensure pain/suffering is relieved.

