The use of complementary and alternative medicine therapy has increased in the human population over time. Approximately 40% of the USA adult population were taking supplements on a regular basis in 2000, in comparison to only 28.7% in 1987 (Millen et al, 2004). In the UK, sales of herbal remedies increased from £27 million in 1991 to £38 million in 1996 with an estimated 25% of the UK population using complementary and alternative medicine therapies (Ritchie, 2007). A survey study of pharmacy customers in Australia reported that 72% had used a complementary medicine in the previous 12 months (Braun et al, 2010).
Many people who purchase supplements are pet owners, so it is not surprising that there are now several supplements designed for dogs and cats. According to a study, almost 30% of pet owners in the UK feed supplements to their pets (Thomson et al, 2008), however, the feeding of supplements in Australia and the USA is lower, with approximately 10–13% of owners giving supplements to their pets (Freeman et al, 2006; Laflamme et al, 2008).
The use of several different supplements has been reported in pets, including chondroitin, glucosamine, fatty acids/oils, aloe vera, milk thistle, probiotics, multivitamins, vitamin C, calcium, yeast, enzymes, herbs, garlic and specific amino acids (Freeman et al, 2006; Thomson et al, 2008). Chondroprotective agents, such as glucosamine and chondroitin, fatty acid supplements and probiotics are among the most commonly used supplements for dogs and will be discussed in this article.
Glucosamine and chondroitin
Osteoarthritis (OA) is a painful condition involving synovial inflammation and breakdown of articular cartilage, and in dogs is the most commonly observed non-traumatic orthopaedic condition in the UK (Clements et al, 2006). It is estimated that 20% of dogs over 1 year of age and more than 90% of dogs over 5 years of age are affected by OA (Clements et al, 2006). Clinical signs typically include difficulty rising from rest, stiffness and a reluctance to walk, run, jump or play (Towell and Richardson, 2010).
Conventional therapies traditionally include the use of non steroidal anti-inflammatory (NSAID) medications to alleviate the inflammation and pain. In the case of NSAIDs, the long-term side effects, which include hepatic toxicity and gastrointestinal ulceration, have contributed to the increased interest in alternative therapies (Canapp et al, 1999; McCarthy et al, 2007).
Despite numerous studies on glucosamine and chondroitin in several species, little is known about how these nutrients affect cartilage metabolism within the joint. There is also still debate over whether glucosamine hydrochloride has a higher bioavailability than glucosamine sulphate, as ionization by endogenous stomach hydrochloric acid yields the same glucosamine derivatives from both glucosamine salts (Adebowale et al, 2000). This may indicate that the products which contain glucosamine hydrochloride are more purified than those containing glucosamine sulphate, which is why a higher dose is required for glucosamine sulphate products.
A study in dogs that examined the bioavailability of a glucosamine hydrochloride supplement after single and multiple doses, showed a bioavailability of 10–12% for glucosamine hydrochloride irrespective of dosing regimens (Adebowale et al, 2002). This study suggested that previous articles, which reported a bioavailability of 26%, most likely overestimated the bioavailability as they did not differentiate between the drug and the inactive metabolites. In the same study by Adebowale et al (2002), the bioavailability of a low molecular weight chondroitin sulphate supplement in dogs was investigated and found to be approximately 5.0% after a single dose, which increased with multiple dosing to over 200% due to the accumulation of total disaccharides in the plasma. This indicates that multiple doses of chondroitin sulphate are required to achieve adequate bioavailability in dogs.
Most studies in dogs use subjective means of assessment of efficacy with small sample sizes of animals or utilize in vitro techniques (Towell and Richardson, 2010). One study that used an objective measurement of force plate analysis, which measures the amount of weight that an animal will place on the limb during movement, did not detect a significant improvement in dogs treated with glucosamine hydrochloride and chondroitin sulphate within 60 days (Moreau et al, 2003). Another study using both glucosamine and chondroitin did detect a significant improvement, but only after 70 days using subjective arthritis scores by the veterinarian and quality of life scores by the owner (McCarthy et al, 2007). The improvement in bioavailability of chondroitin after multiple doses and the improvement in subjective scores after 70 days could indicate why other studies with shorter end points did not yield significant results. This also indicates that if glucosamine and chondroitin are used as part of a therapeutic regimen, short-term management of pain and inflammation will be required before the supplement is likely to have an effect. Further studies are required to establish the efficacy of glucosamine and chondroitin over a longer period of time, however, a change in study approach is needed to achieve significant results that will be applicable to clinical practice (Heyland, 2001).
Furthermore, the quality and safety of glucosamine and chondroitin supplements are under question. One study demonstrated that the level of the active ingredient specified on the label may not be accurate, as the glucosamine and chondroitin products studied contained levels between 0% and 115% of the label claim (Adebowale et al, 2000). In a best case scenario this indicates that the owner is potentially wasting money on an ineffective product, at worst this could mean the animal may receive enough of the active ingredient to cause a toxicity syndrome. This indicates that these products should be used with caution as two of the 35 dogs (6%) in the study by McCarthy et al (2007) had adverse reactions, presenting as gastrointestinal upset. Additionally there have been reports to the American Society for the Prevention of Cruelty to Animals, Animal Poison Control Centre of hepatic toxicity from overdose with joint supplements in 21 dogs (Khan et al, 2010). Hence, in a clinical context it is always advisable to contact the company to ask them how they determine that their product contains the levels claimed on the label, and to monitor the patient for signs of improvement or adverse reactions when using these supplements.
Green lipped mussel (GLM) (Perna canaliculus)
GLM contains a combination of omega-3 fatty acids, chondroitin sulfates, amino acids, vitamins E and C and minerals (Bierer and Bui, 2002). It is thought that the main action of the GLM is the anti-inflammatory properties of the omega-3 fatty acids (especially ecosatetranoic acid), although other constituents may have chondroprotective properties (Hielm-Björkman et al, 2009). Heat processing destroys the activity of the GLM powder, which means that veterinary therapeutic foods that include this supplement need to use low-temperature manufacturing or apply the GLM coating after the heat processing of the diet (Bierer and Bui, 2002).
A similar efficacy of approximately 30% reduction in subjective arthritis scores was demonstrated in a study by Bierer and Bui (2002) with sample sizes of approximately 30 animals for a powdered supplement, a supplemented treat and a supplemented diet when a similar dose of GLM was administered (750 mg/day for 34–25 kg dogs, 450 mg/day for <25 kg dogs). Similar problems are encountered with the literature for the efficacy of GLM as for glucosamine and chondroitin. Studies tend to be subjective with moderate numbers of animals and ill-defined end points. Most studies for GLM detect some improvement but recommend that longer studies are needed (Bierer and Bui, 2002; Pollard et al, 2006; Servet et al, 2006; Hielm-Björkman et al, 2009).
Polyunsaturated fatty acids (PUFAs)
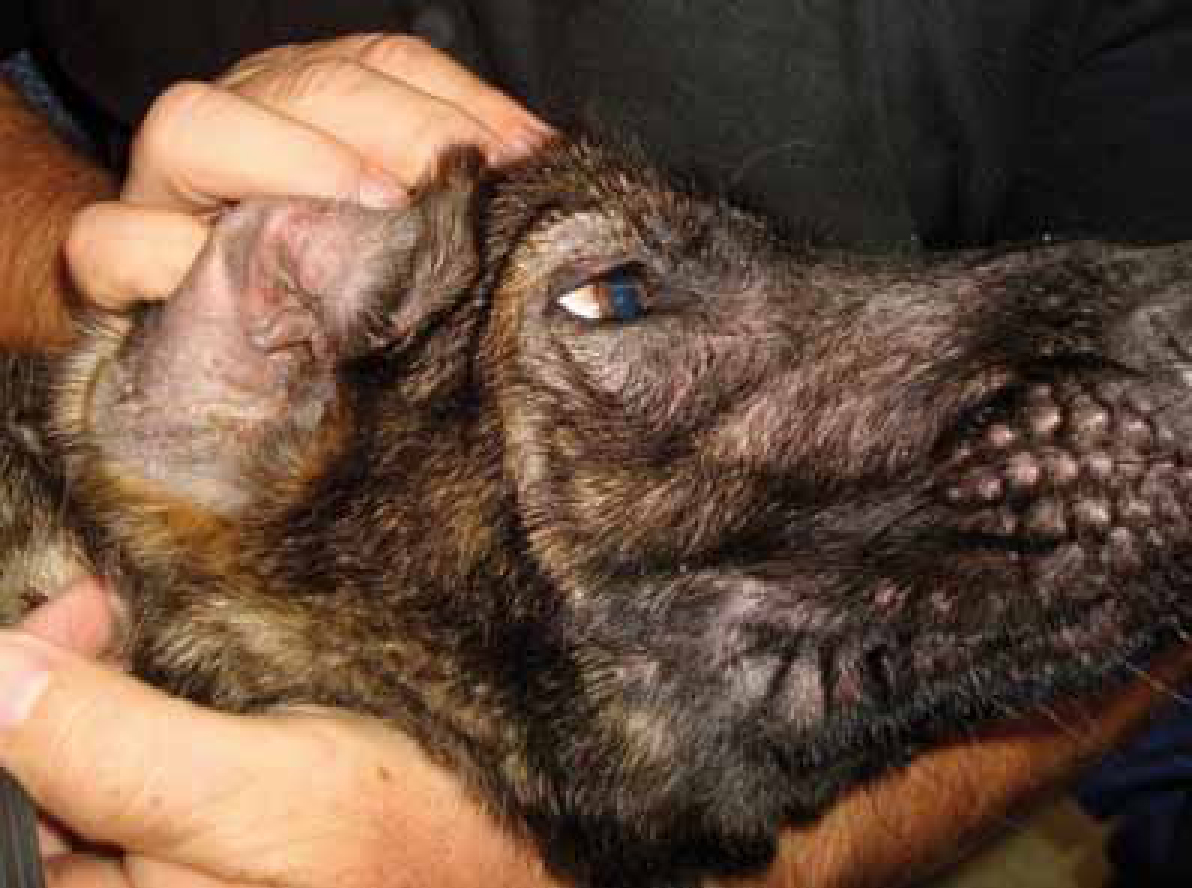
The effects of supplementation of the diet with PUFAs have been studied for many different conditions, including atopic dermatitis (Figure 1). Due to this extensive research, a full discussion of their use is beyond the scope of this article. A summary of the conditions in which PUFAs have been studied is provided in Table 1.
| Condition | Mode of action | References |
|---|---|---|
| Inflammation | EPA and DHA supplementation reduces PGE2, IL-1 and IL-6 concentrations in serum | (Wander et al, 1997) (LeBlanc et al, 2008) |
| Osteoarthritis | Omega-3 supplementation reduces the production of enzymes that degrade cartilage, decreases inflammatory cytokines and may allow a reduction in carprofen dosage | (Curtis et al, 2000) (Hansen et al, 2008) (Fritsch et al, 2010) |
| Renal insufficiency/failure | Omega-3 supplementation decreases ischaemic failure and progression of renal disease | (Neumayer et al, 1992) (Brown et al, 1998) (Brown et al, 2000) |
| Cardiovascular disease | Omega-3 supplementation decreases IL-1 serum concentration and reduces cachexia Omega-3 acts as an anti-arrhythmic | (Freeman et al, 1998) (Billman et al, 1999) (Kang and Leaf, 2000) |
| Cognitive function | DHA is associated with normal neurological function Supplementation may restore the membrane concentrations of DHA that decline in cognitive dysfunction associated with ageing | (Heinemann et al, 2005) (Youdim et al, 2000) |
| Neoplasia | Omega-3 supplementation reduces inflammation, plasma lactic acid response and increases survival time in lymphoma | (Ogilvie et al, 2000) |
| Atopic dermatitis | Omega-3 supplementation decreases inflammation Omega-6 supplementation potentially improves skin barrier function | (Olivry et al, 2001) |
EPA, eicosapentaenoic acid, an omega-3 PUFA; DHA, docosahexaenoic acid, an omega-3 PUFA; PGE2, prostaglandin E2, an inflammatory arachidonic acid metabolite; IL, interleukin, an inflammatory cytokine
A high level of PUFA supplementation has been studied for some conditions, for example 10 000 mg/1000 kcal omega-3 for OA (Fritsch et al, 2010). When attempting to achieve a level this high using a fish oil supplement with a regular adult maintenance diet, the risk of unbalancing the diet increases. The PUFA content of many commercially produced diets has been altered in light of the current PUFA supplementation studies (Roudebush, 2008). The benefit of using one of these commercial diets with a higher selected PUFA content is the reduced risk of the diet being unbalanced, as the company producing the diet can allow for the higher PUFA content when balancing the diet. Al-though the PUFA supplemented diets show promise, further research is still required to determine more accurate PUFA levels for clinical efficacy and the optimal omega-3 to omega-6 ratio for different conditions (Roudebush, 2008).
Probiotics
Probiotics are live organisms that are believed to have health benefits, such as improving the intestinal flora, enhancing the immune system (Wynn, 2009), and improving the nutritional value of food (McCoy and Gilliland, 2007), when administered orally (Blood et al, 2007). Prebiotics differ from probiotics as they are substrates, primarily indigestible carbohydrates, that promote the growth of bacteria that are considered to be beneficial (Blood et al, 2007), thus limiting the growth of pathogenic bacteria. Probiotics have been used in human nutrition, primarily in dairy products, and in production animal nutrition for over 10 years. More recently, companion animal nutrition has been identified as another potential area for their use (McCoy and Gilliland, 2007; Lauková et al, 2008).
Strains of bacteria are considered as a potential probiotic if they can resist digestion and survive through the gastrointestinal tract, colonize the gastrointestinal tract and have an antagonistic effect against pathogens (Lauková et al, 2008). Some potential probiotic strains have been identified in the literature for canines, consisting of primarily Enterococcus spp. and Lactobacillus spp. (McCoy and Gilliland, 2007; Laukova et al, 2008). A summary of the findings of the use of different probiotic strains in dogs is shown in Table 2.
| Probiotic strain | Dose (CFU/day) | Effect on GI microbiota | Other findings | Reference |
|---|---|---|---|---|
| Enterococcus faecium (EE3) | 2 × 109 | ↑ Lactic acid bacteria | No adverse effects reported by owners | (Marci˘náková et al, 2006) |
| ↓ staphylococci | ||||
| ↓ Pseudomonas-like bacteria | ||||
| No effect on E. coli | ||||
| E. faecium NCIB 10415 | 9.2 × 109 | ↓ Clostridium spp. No effect on | No other findings | (Vahjen and Männer, 2003) |
| Salmonella spp. or Campylobactor spp. | ||||
| E. faecium (SF68) | 5 × 108 | Effect on GI | ↑ faecal IgA | (Benyacoub et al, 2003) |
| microbiota not studied | ↑ circulating canine distemper virus vaccine-specific IgG and IgA concentrations | |||
| E. faecium (SF68) | 5 × 108 | Effect on GI | No effect on giardial cyst shedding | (Simpson et al, 2009) |
| microbiota not studied | Did not alter adaptive or innate immune responses | |||
| Lactobacillus animalis (LA4) | 5 × 108 | ↑ Lactobacillus spp. | Faecal concentrations of ammonia and SCFA were not influenced | (Biagi et al, 2007) |
| ↓ Entercoccus spp. | ||||
| ↓ Clostridium perfingens (in vitro) | ||||
| Lactobacillus acidophilus (DSM13241) | 109 | ↑ Lactobacillus acidophilus | Able to survive manufacture and storage when freeze-dried | (Baillon et al, 2004) |
| ↑ Clostridium spp. | ||||
| L. acidophilus (DSM13241) | Added to diet post extrusion at 6 x106 CFU/g | ↑ Lactobacilli | Improved faecal consistency and defecation frequency | (Pascher et al, 2008) |
| ↑ bifidobacteria | ||||
| ↓ Clostridium perfringens | ||||
| ↓ Escherichia spp. |
CFU, colony forming units; GI, gastrointestinal; SCFA, short-chain fatty acids
The findings included in Table 2 indicate that different effects on the microbiota of the gastrointestintal tract can be expected between different strains of probiotic bacteria. Furthermore, a significant reduction of the pathogenic bacteria in healthy dogs was not achieved in two of the studies (Biagi et al, 2007; Pascher et al, 2008), and one study showed a variable effect on the microbiota of healthy dogs (Vahjen and Manner, 2003). Both Vahjen and Manner (2003) and Pascher et al (2008) question whether there is a beneficial effect from the use of probiotics in healthy dogs for this reason, but did propose that dogs with diarrhoea or undesirable faecal consistency may benefit from their use. Therefore, further studies are required to determine the effect of probiotics in dogs with diarrhoea.
For a probiotic product to be effective, it needs to contain live probiotic bacteria in an effective dose. A study by Weese and Arroyo (2003) found that none of the 19 diets with a probiotic claim on the label in their study contained all the organisms listed on the label, with only approximately 50% of the products containing one or more of the listed organisms. It is, therefore, advisable to determine how the company producing the supplement or supplemented diet ensures that the microorganisms contained in the product are at therapeutic levels prior to use.
The safety of probiotics has not been reported in companion animals, however, bacteraemia and septicaemia have been reported in humans in relation to probiotic treatment during severe illness and so caution is advised for patients that are severely immunocompromised (Wynn, 2009).
Evaluating supplements
Supplements generally slip through the regulatory cracks as they are considered to be neither a food nor a drug (Bauer, 2001). Regulatory organizations, such as the National Animal Supplement Council in the USA, have been formed to bring some regulation to this industry, so it is advisable to use supplements from companies that are members of this type of organization, and which follow their guidelines. Due to this lack of regulation, it falls to the veterinary staff to evaluate each supplement prior to use or recommendation for patients.
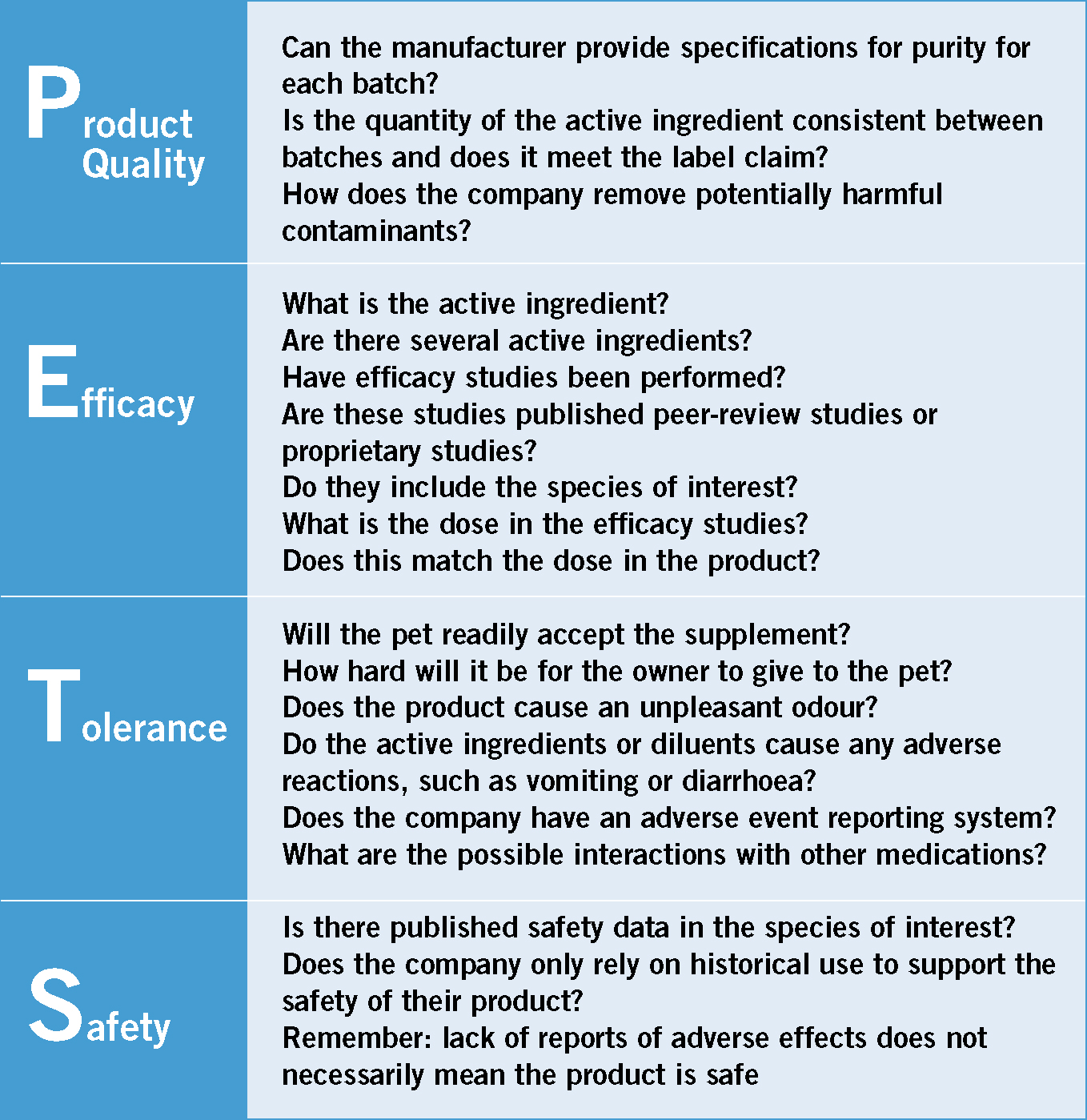
Bauer (2001) has presented a structure for evaluating supplements with an easy to remember acronym: PETS (Figure 2). PETS stands for the four areas of evaluation that should be addressed which include: product quality, efficacy, tolerance and safety (Bauer, 2001). The questions that should be asked of a product related to each area of evaluation are provided in Figure 2.
Conclusions
The use of supplements to aid in the healthcare of dogs may be beneficial, however, the safety and efficacy of these products must be determined before recommending them for use. It is advisable to always obtain an accurate diet history, including whether supplements are being fed to the pet. The animal's base diet should then be evaluated, and if necessary, modified before considering additional supplementation.
