Intensive nursing often means taking care of patients that have a multitude of lines attached to them (Figure 1). Whether that be intravenous catheters, wound drains or feeding tubes, it is important to bear in mind that any extra tube or drain on a patient is another entry point for bacteria to multiply. Hospital acquired infections account for up to 40% of increased length of stays for patients in the intensive care unit (Ershova et al, 2018); and nosocomial infections increase morbidity and mortality as well as the length of stay in hospitals and in turn will in-crease cost to the client. It has been shown that good hygiene and aseptic techniques in nursing can improve infection rates by up to 40% in some studies (Mukhopadhyay, 2018).
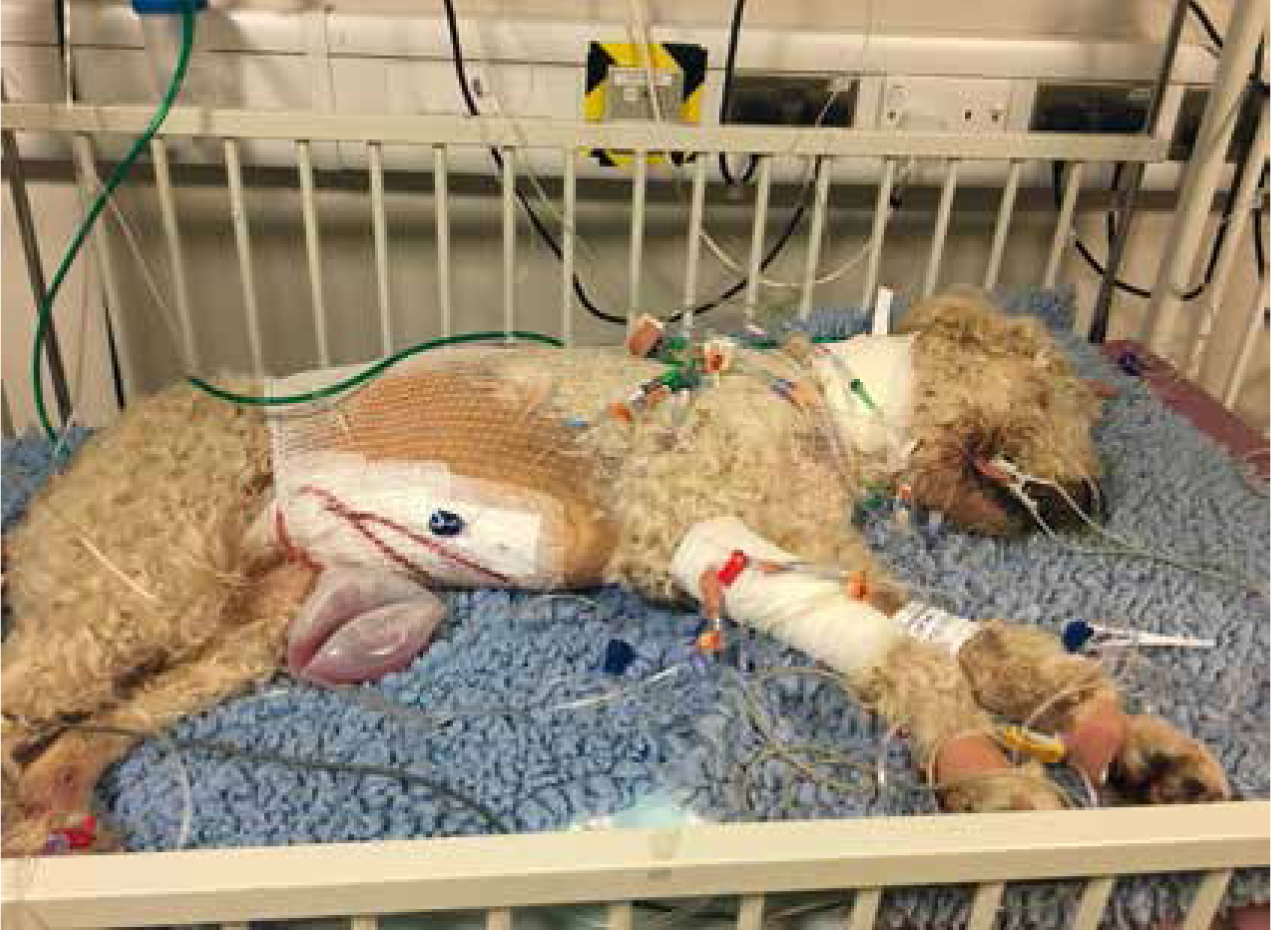
With multidrug resistant bacteria on the rise, and an ever-limited choice of antibiotics, it has never been more important to focus on infection control in practice. With every antimicrobial available there is documented resistance to it, and antimicrobial resistance has led to the increase in morbidity and mortality from infectious conditions (Reygaert, 2018). Infection control is everyone's responsibility. Veterinary nurses are likely to be in more direct contact with patients than veterinary surgeons — this has been shown in human medicine where on average 67% of nurses spent over 20 minutes with some patients compared with 29% of doctors (English et al, 2018). With this in mind, veterinary nurses should make every effort to maintain gold standard hygiene.
The World Health Organisation (WHO) method of handwashing is commonly taught early on in all veterinary training programmes. It should, therefore, be common practice across the profession. It is recommended that if there is gross contamination of organic material on the hands (urine, blood etc), hands should be washed with detergent and water (wetting the hands first, then using the soap to lather and complete the WHO method, over 60 seconds (WHO, 2018)).
Between patients, when hands are not soiled, it is recommended to use an alcohol rub over a 30 second timeframe (WHO, 2018). There are many alcohol rubs on the market for use in medical industries. Sterilium is popular and can be used multiple times throughout the day. Many surgeons now use alcohol rubs to do a surgical hand rub (SHR) rather than a surgical hand scrub (SHS). It has been shown that a SHR has similar efficacy, but with increased longevity, and that it also reduces cost (Tavolacci et al, 2006).
Gloves should always be used if coming into direct contact with bodily fluids, for example, when placing a line, or indirectly, for example when emptying a urinary bag. However, gloves should not be used as a replacement for good hand hygiene!
Barrier nursing
Barrier nursing describes the care of patients with infectious diseases, however, barrier nursing should be standard in all practices for many of the patients in the hospital. Patients will need to be barrier nursed if they carry a contagious disease or have a multidrug-resistant bacteria such as meticillin-resistant Staphylococcus aureus (MRSA). In addition, some patients require reverse barrier nursing for protection of themselves. This could be because they are receiving immunosuppressant drug therapy, or because they have neutropenia.
Patients with infectious diseases are best nursed in an isolation facility, however options are limited when that patient is also critically unwell and requires intensive care.
Intravenous catheters (IVCs)
Intravenous catheters are one of the biggest sources of nosocomial infections in veterinary patients (Jones et al, 2009). Correct placement technique is as follows:
Long stay catheters
Long stay IVCs may be placed centrally into the jugular vein (central venous catheter (CVC)) (Figure 4) or via the saphenous vein (peripherally inserted central catheters (PICC)). They are used for a variety of reasons, for example, for patients that are going to be hospitalised for a long time as they can be left in place much longer than peripheral IVCs; and in patients that require multiple blood draws during their stay, because regular samples can be taken from patients non-invasively.
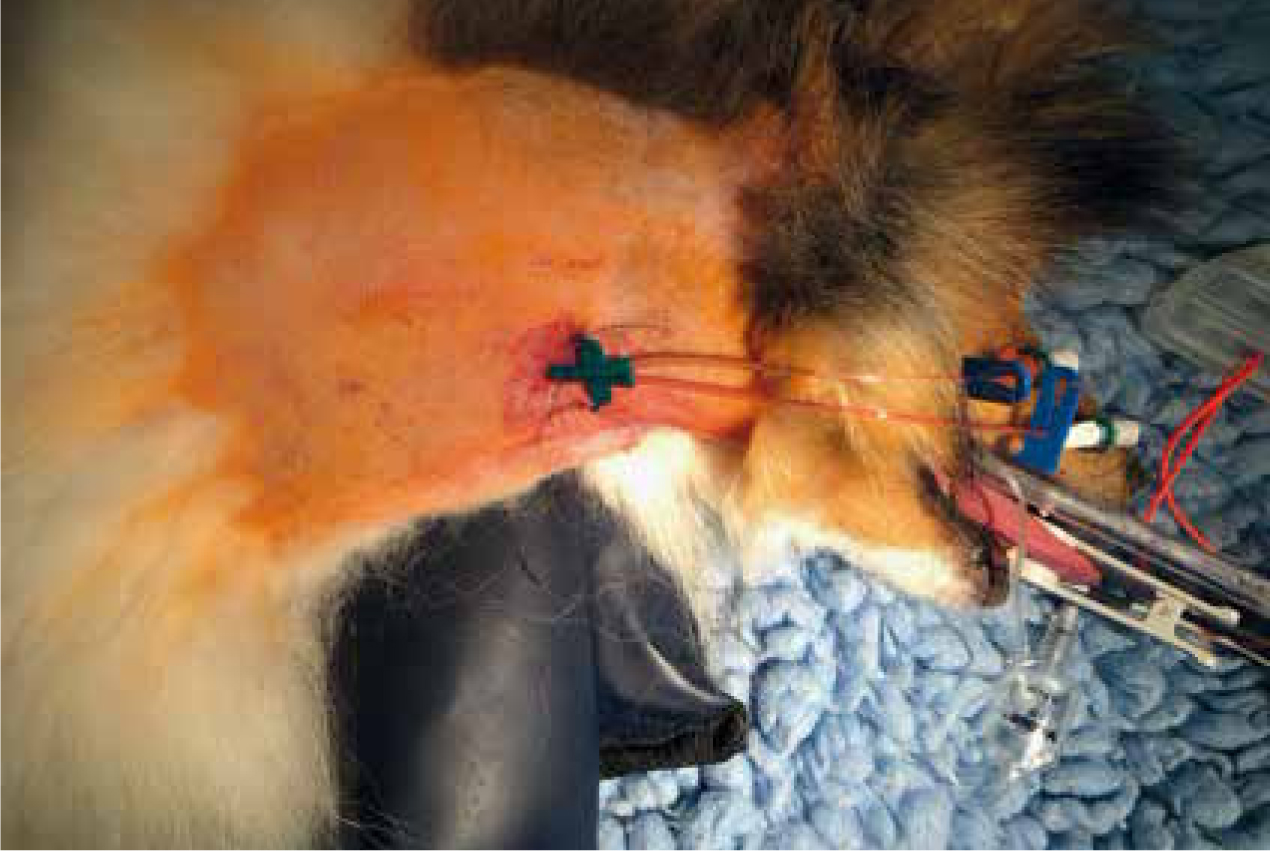
Indications for CVCs include: larger volumes or rates of fluid therapy, and higher concentrations of medications in constant rate infusions (CRIs) that may be more irritant to perivascular tissue. They are also used for monitoring of central venous pressures, and to give access for extracorporeal therapy, such as plasma exchange or renal replacement therapy (Welsh, 2017).
The placement of CVCs is contraindicated in patients with a coagulopathy, raised intracranial pressure, thrombosis, or a contaminated site/skin condition over the vessel, amongst others (Smith and Nolan, 2013).
Urinary catheters
Urinary catheters are placed in practice for many reasons. Often they may be used to measure urinary output over a set timeframe in patients with kidney disease, or they may help in the management of recumbent large dogs.
The same hygiene standards should be applied for placement of indwelling catheters and for those placed intermittently. Correct placement technique includes wearing sterile gloves, and keeping all the equipment and lubricant sterile for the whole procedure.
Indwelling catheters should always be attached to a closed collection system. It is not suitable to have a patient catheterised and free flowing for a number of reasons:
Faecal catheters
Faecal catheters are not common practice, but have been available in the human medical world for a long time now. They can be invaluable in nursing patients with continuous diarrhoea, such as parvovirus patients, or in patients with haemorrhagic diarrhoea syndrome (Figure 5). There are faecal catheters available to buy, but it is also possible to use Foley indwelling catheters in a larger size to the same effect.
When placed into the colon, the balloon will need inflating with slightly more saline than if it was to be indwelling in a urinary bladder. This should always be done following veterinary surgeons' advice. Once in place, the balloon should be deflated, and catheter moved before re-inflating several times a day to help prevent any ischaemia of the colon.
A closed collection system is used, and this enables liquid faeces to be collected and contained.
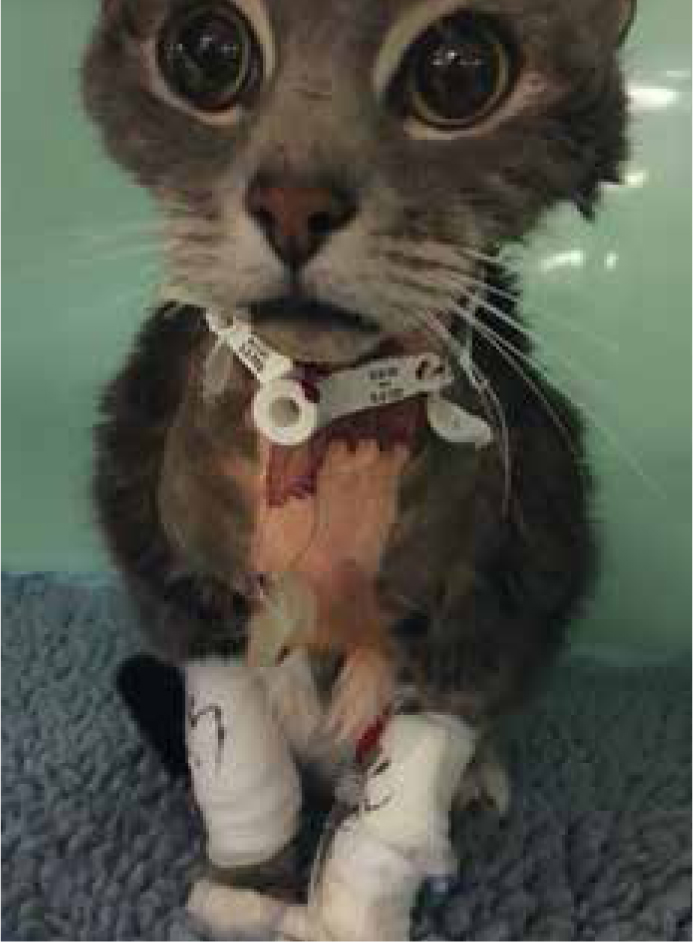
Wound drains
Wound drains should be managed aseptically, and it is standard for any patient with an indwelling drain to be barrier nursed for their safety. All drains, both passive and active are shown to be a risk for infection via bacterial migration up the drain itself (Durai et al, 2010).
Passive drains, such as a Penrose drain, should always be covered with a dressing, either a primapore or something more absorptive (Figures 6 and 7). These may need to be changed very regularly, depending on how productive the drain is. They should be checked regularly, 2 to 4 hourly in the author's hospital, and changed before strike through is seen. Once a dressing has strike through, the protective barrier is compromised, and bacteria can easily cross through the soaked material to the wound.
Active wound drains (Figure 8) should be managed aseptically and emptied regularly. Drains such as Jackson Pratts can be swabbed with an alcohol swab before and after emptying, and it may be possible, depending on the size of the drain used, to connect a needle free valve, thus reducing the chances of infection even further. These can then be drained using syringes to measure the output.

Chest drains
Chest drains are placed for management of effusions or air inside the thoracic cavity. They must be placed by a veterinary surgeon, but can be maintained by registered veterinary nurses under schedule 3 (RCVS, 2018).
Chest drains should be handled aseptically. Sterile gloves should be worn whenever they are to be emptied. Chest drains can be drained intermittently, or continuously (Figure 9) via a continuous suction device (Figure 10) such as a Thoraseal (Cardinal Health) or Thopaz (Medela). These are best used on patients with a continuous pneumothorax, as these patients can decompensate quickly, and it can be challenging to drain them quickly enough manually.

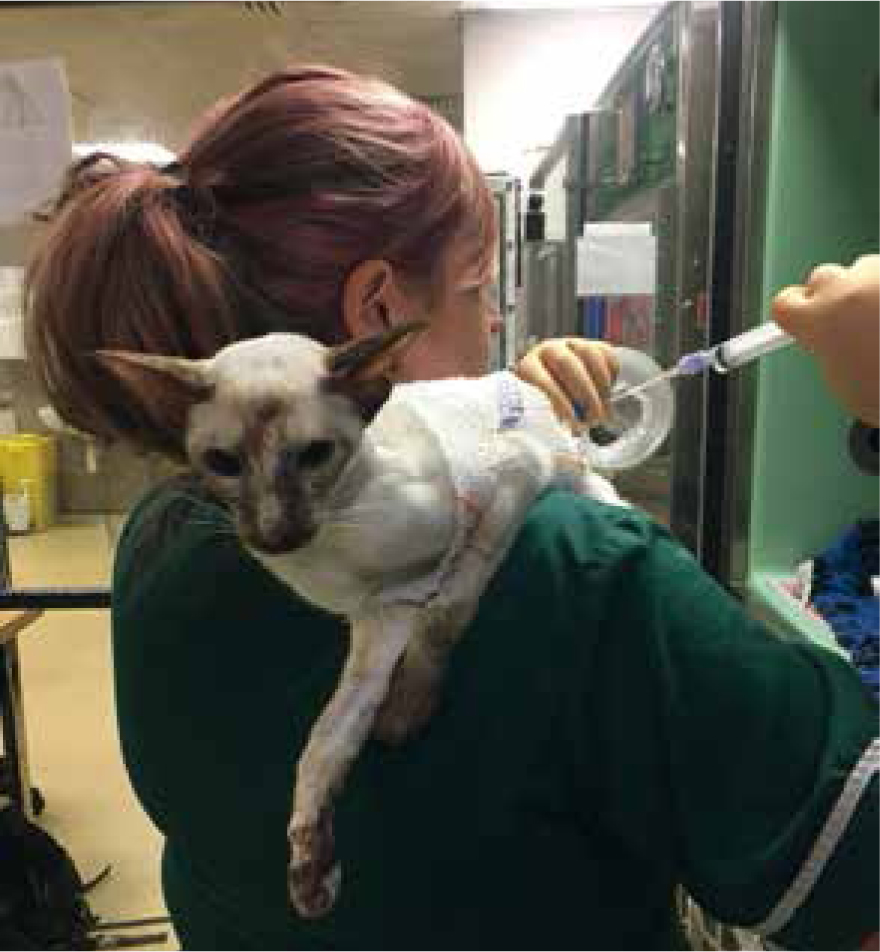
If draining intermittently, ensure the patient is comfortable and calm. Some patients are very tolerant (Figure 11), but some may need to be gently restrained for the procedure. Sterile gloves should be worn, and sterile syringes, injection ports and alcohol swabs should be used.
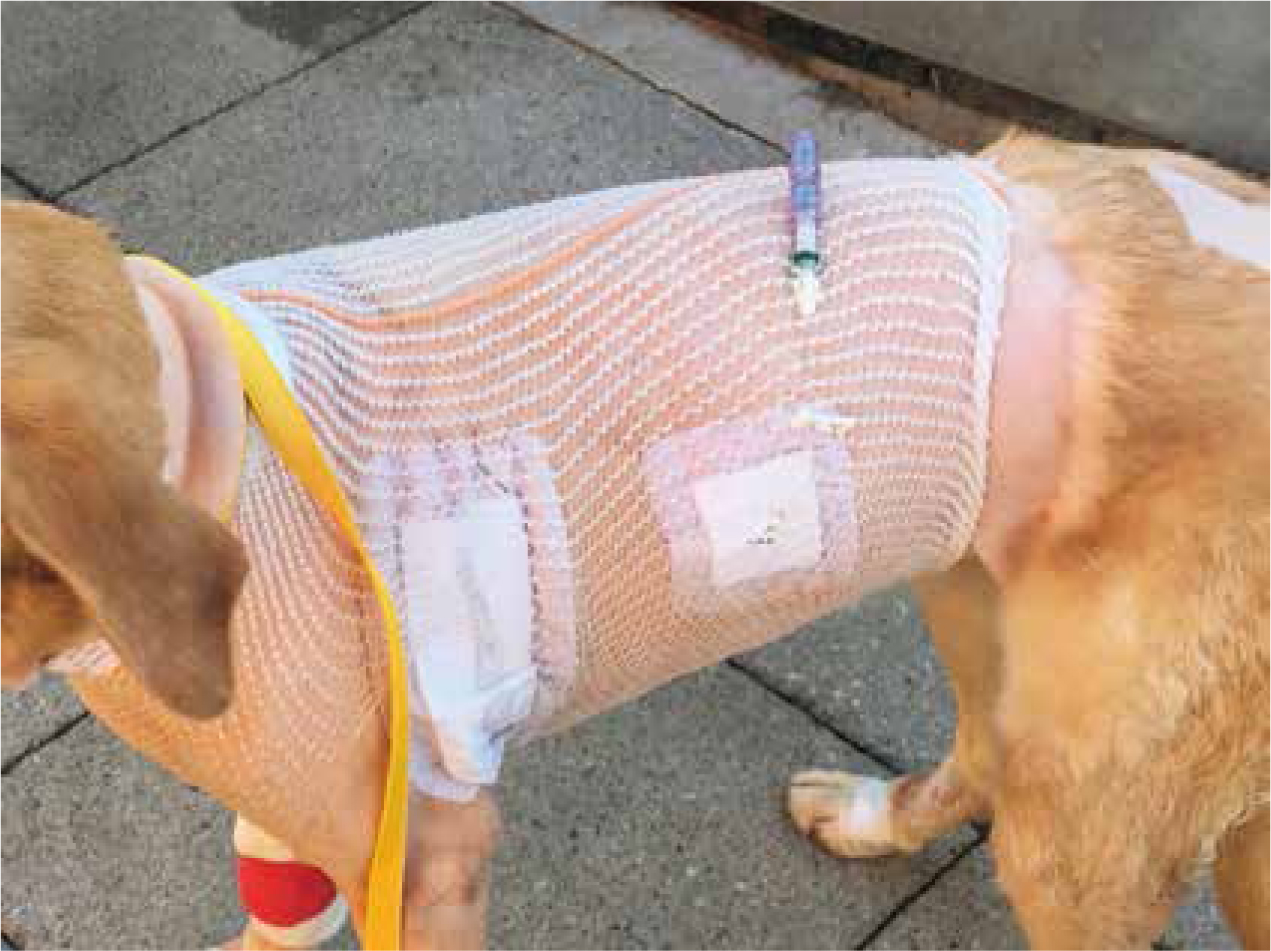
All chest drains should be dressed with a sterile dressing, and either a stocking vest or a buster collar applied to prevent patient interference (Figure 12).
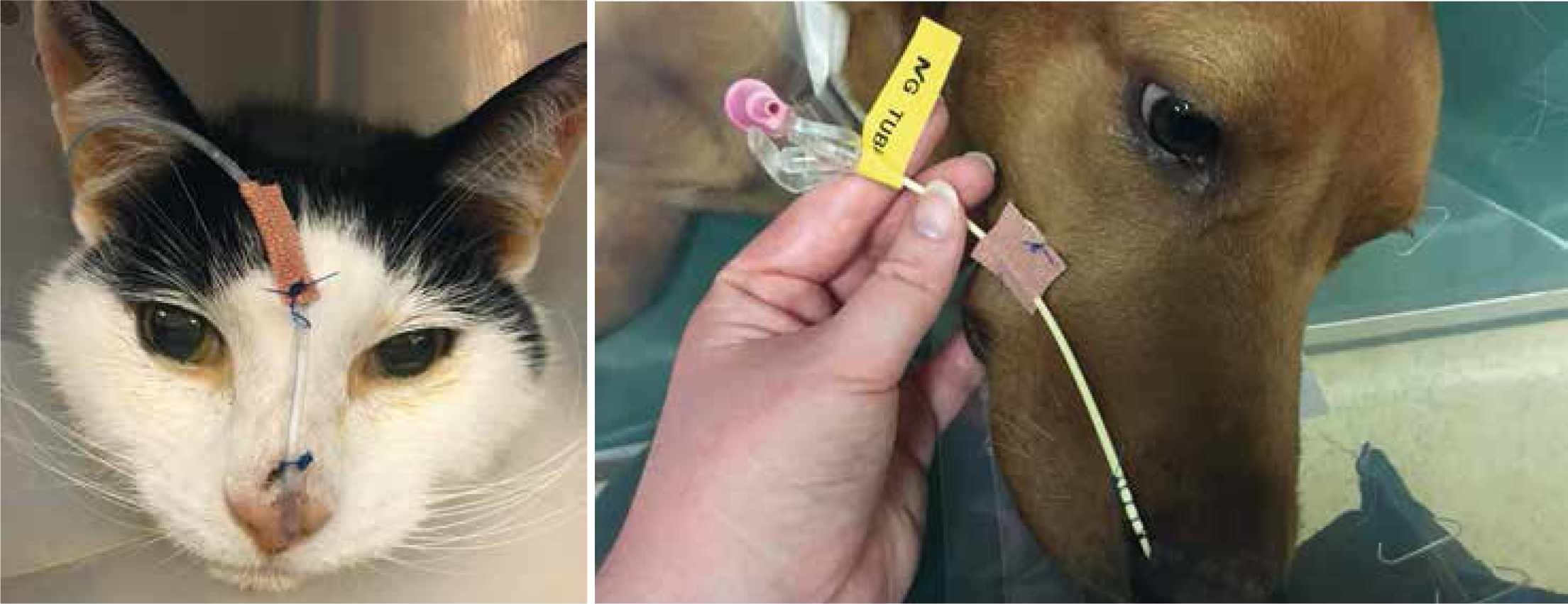
Feeding tubes
There are a variety of feeding tubes available in veterinary practice. RVNs are able to place naso-oesophageal (NO) or nasogastric (NG) tubes; these are the least invasive tubes to place and are often well tolerated in critical patients. Placement should be aided by lubricant and local anaesthetic drops. Tubes should be placed into the nasal cavity ventromedially. The patient should swallow the tube which will aid placement and indicate that the tube is in the oesophagus. The tubes should be secured in place either with sutures or staples. Once in situ, the placement can be confirmed via a chest radiograph. Other ways of checking placement are by placing a capnograph onto the end of the tube (if the tube is in the airway you will get a CO2 reading), or by aspirating the tube (correct placement is confirmed if gastric residuals are suctioned from an NG tube).
All feeding tubes should be labelled as such to avoid any confusion (Figure 13).
Tracheostomy tubes
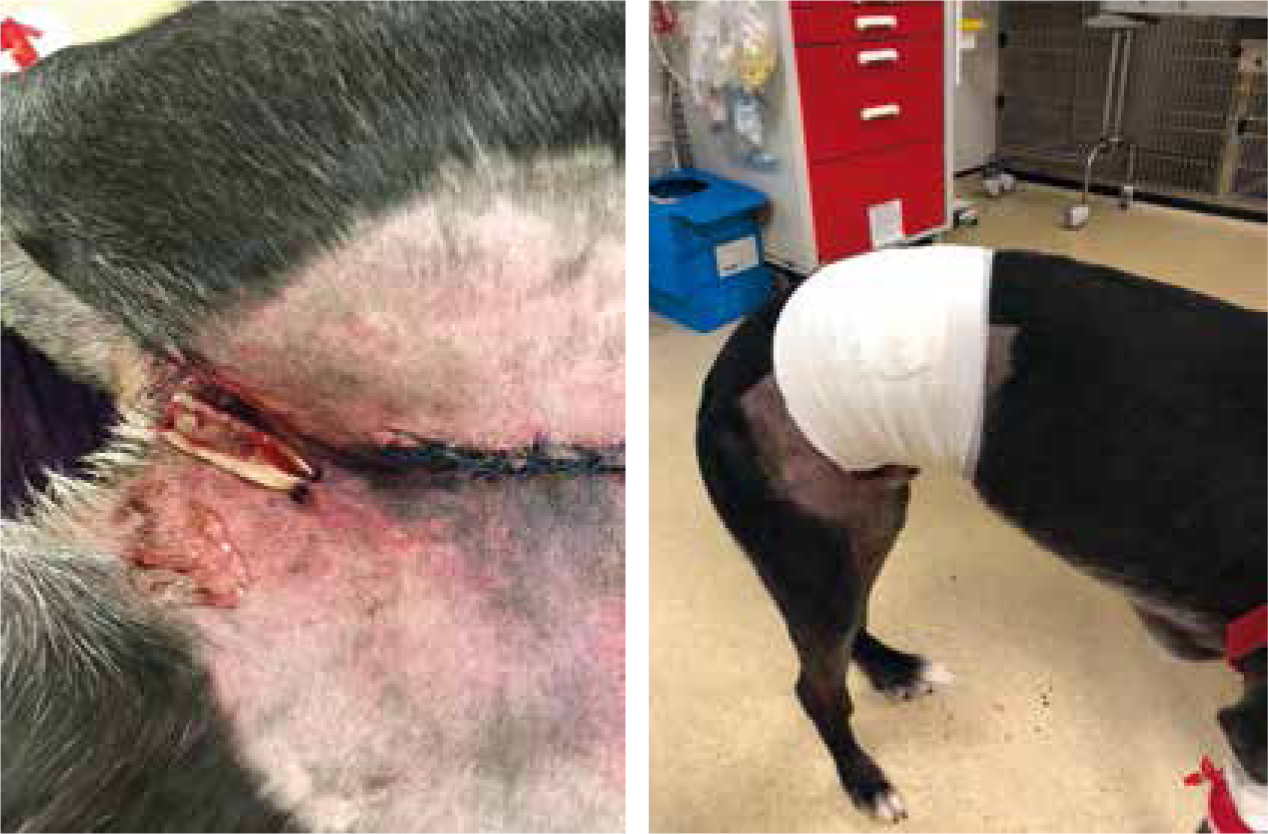
Tracheostomy tubes are placed in patients that may have a compromised upper airway. They may be placed in patients with laryngeal paralysis, brachycephalic obstructive airway syndrome (BOAS) (Figure 14), or if there is an obstructive mass in situ (neoplastic or infectious; Figures 15 and 16).

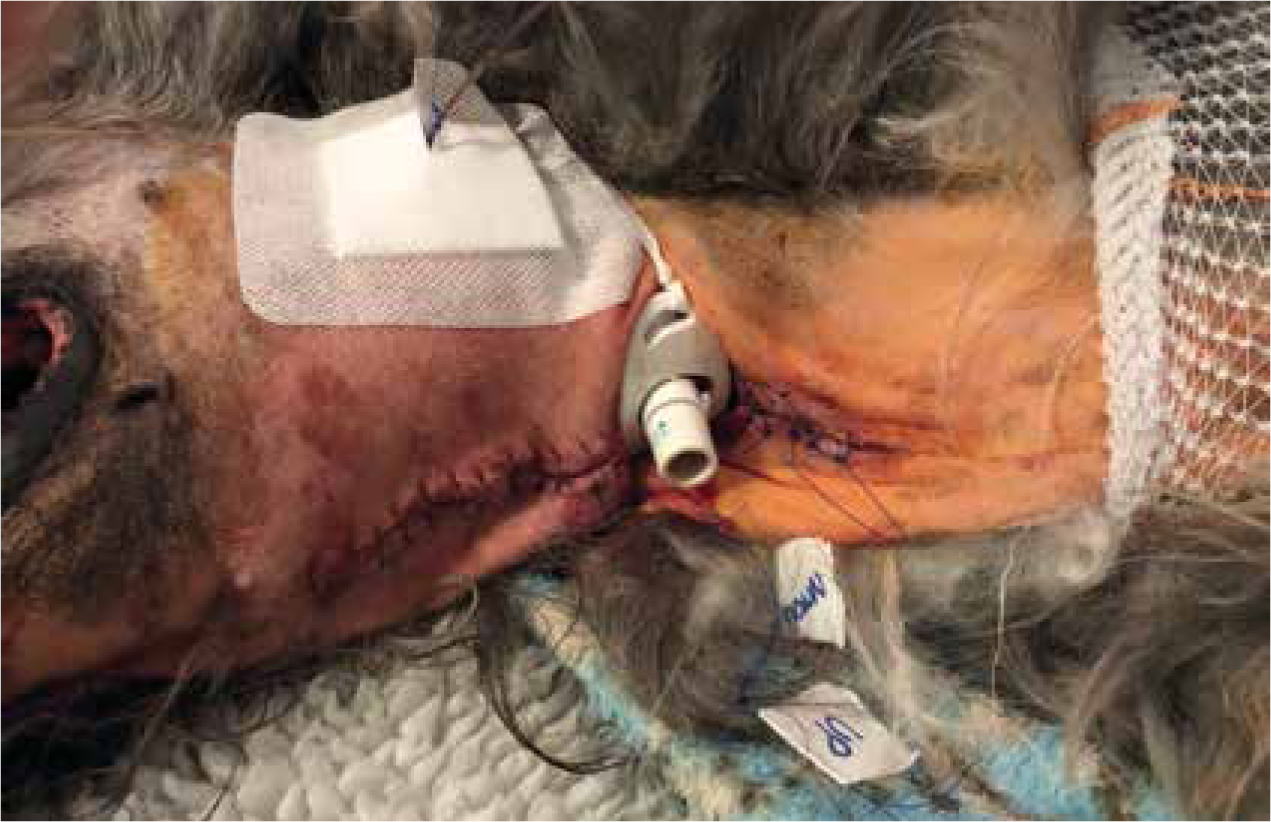
Studies have shown high rates of complications in tracheostomy tubes in dogs (86% of cases between 1998–2007) (Nicholson and Baines, 2012). These are effectively an open stoma into the airway of these patients, so infection control is important (Table 1).
| Procedure | Techniques | Sterile gloves | Non-sterile gloves | Apron | Comment |
|---|---|---|---|---|---|
| Changing of tracheostomy tube | Aseptic technique | x | √ | √ | Initial insertion must be in theatre using sterile surgical aseptic technique unless life threatening emergency |
| Tracheostomy care in-patient setting, e.g. dressing changes, endotracheal suctioning | Aseptic technique | x | √ | √ | |
| Tracheostomy care in home setting, e.g. dressing changes, endotracheal suctioning | Clean technique if over 72 hours old | x | √ | √ |
All patients require preoxygenation prior to any tracheostomy care, as by doing this you may be obstructing the airway. Regular cleaning of the stoma site should be undertaken at least 4 hourly, more frequently if the stoma is productive. Non-sterile gloves should always be worn on handling the tracheostomy tube/cleaning of the stoma. Non-sterile gloves can be worn for aseptic cleaning techniques as long as the inner cannula is not touched directly, as advised by NHS England (Hunt, 2014). Aseptic non touch technique (ANTT) is used in human hospitals throughout the UK (ANTT, 2018), and provides guidelines to medical professionals on the use of aseptic techniques from surgery to home care of stomas.
If the tube has an inner cannula, this can be removed at frequent intervals and flushed to clean — nothing should be placed down the tracheostomy tube as this may cause abrasions on the inside of the tube, which will increase the susceptibility for mucous to stick, causing an obstruction. The same is true if the tube requires suctioning. If a tube requires suctioning, ensure that the length of the suction tubing does not exceed the length of the tracheostomy tube as this may damage the mucosal lining of the trachea.
Care must be taken in recumbent patients to prevent them from occluding their tracheostomy tube by lying on it, as asphyxiation is possible and has been shown to occur in 1–39% of patients (Nicholson and Baines, 2012).
Conclusion
In conclusion, hospital acquired infections account for increased length of stay for patients in the intensive care unit (Ershova et al, 2018), and nosocomial infections increase morbidity and mortality. However, it has been shown that good hygiene and aseptic techniques in nursing can improve infection rates (Mukhopadhyay, 2018). By adhering to the above standards of implementation of care, ensuring good hand washing technique and becoming more aware of infection control, it is possible to nurse patients with tubes, lines and drains safely amongst others in the veterinary hospital.

