Seizures can present to veterinary practice as an emergency with an actively seizuring patient, or as a consultation following seizures at home. Seizures can be upsetting for owners to witness, so nursing care can often extend to the client as well as the patient. Because of the wide variety of underlying causes for a seizure, there are a wide variety of nursing interventions that can be implemented, making the role of the registered veterinary nurse (RVN) vital in providing a suitable and holistic level of care to the patient. Ultimately the goal of nursing care and veterinary treatment is to allow the patient to continue to have a reasonable quality of life; whether this means a complete seizure-free status or merely a reduced frequency or intensity is determined by the client and the veterinary team.
Seizures
Seizure is a broad term, but in veterinary medicine most commonly refers to a clinical manifestation of excessive hyperexcitability in the cerebral cortex (Meland and Carrera-Justiz, 2018). This hyperexcitability of neurons can occur in the entire cerebrum, leading to a generalised seizure, or in one specific lobe or region of the brain, creating a focal seizure (Thomas and Dewey, 2016). Prolonged or recurrent seizure activity and overstimulation of neurons can lead to cellular damage, necessitating the need for prompt treatment (Thomas and Dewey, 2016; Packer, et al, 2017). Classification and description of seizure activity varies hugely among veterinary professionals (Packer et al, 2015), and some of the more common terminology used is summarised in Table 1.
Table 1. Seizure terminology and definitions
| Terminology | Definition |
|---|---|
| Tonic | Generalised muscle hypertonicity or rigidity |
| Clonic | Rhythmical convulsing or paddling of limbs |
| Tonic-clonic | Initial sustained muscle hypertonicity followed by clonic jerking of limbs |
| Myoclonic | Brief muscle contractions that may be generalised or confined to a specific muscle or muscle group |
| Electroencephalography (EEG) | An electrodiagnostic test that measures electrical activity in the brain |
| Reactive seizure | A seizure occurring as a result of the brain's natural response to a disturbance (for example, toxicity or head trauma); when the cause is reversed, the seizures will stop |
| Epileptic seizure | A seizure occurring as a result of intracranial disease |
| Epilepsy | Recurrent seizures occurring over a prolonged period of time |
| Idiopathic epilepsy | Epilepsy with no underlying structural or metabolic disease |
| Cluster seizures | Multiple (two or more) seizures during a single 24-hour period |
| Status epilepticus | A seizure lasting longer than 5 minutes, or cluster seizures where the patient does not fully recover between seizures |
Recent studies show that epileptic seizures occur in between 0.6 and 5% of the canine population (Kearsley-Fleet et al, 2013; Uriarte and Maestro Saiz, 2016; Erlen et al, 2018), with male dogs and certain breeds overrepresented (Heske et al, 2014). Overrepresented breeds include Boxers, Pugs, Basset Hounds, Border Terriers and Border Collies, suggesting a potential genetic cause for epilepsy in certain breed populations (Erlen et al, 2018). Generalised tonic-clonic epileptic seizures are the common emergency presentation in veterinary practice and are characterised by tonic-clonic limb movements, a loss of consciousness, and often an emptying of the bladder and/or bowels. The animal may also present with breathing abnormalities and cyanosis (Thomas and Dewey, 2016).
Focal seizures are much more varied in their presentation depending on the area of the brain involved and are not usually associated with a loss of consciousness. Facial twitching, isolated limb contractions, hypersalivation and behavioural changes, for example fly-catching behaviours, are all examples of focal seizure activity (Packer et al, 2017). In some cases, focal seizures can further evolve into more generalised seizure activity, with the associated motor components and loss of consciousness (Thomas and Dewey, 2016). Sustained focal seizure activity has the ability to cause cellular damage in the same way as a generalised seizure, making recognition and treatment of these seizures just as important as for generalised epileptic seizure activity (Packer et al, 2017).
Epileptic seizures can be defined as having four distinct clinical stages (Podell, 2012):
- The prodrome is the long-term time period prior to the onset of the seizure, where the patient may exhibit behavioural changes such as restlessness, increased anxiety or abnormal vocalisation. This stage can last hours to days before the seizure actually occurs, and some owners may be able to identify these subtle behavioural changes.
- The aura stage occurs immediately prior to the seizure, and may be defined as the start of the seizure itself (Thomas and Dewey, 2016). The initial abnormal electrical activity causes further behavioural changes such as pacing or hypersalivation. The prodrome and aura are differentiated in human medicine by an electroencephalogram (EEG), as abnormal electrical activity is only observed during the aura. Commonly in animals, the prodrome and aura are often referred to as one preictal stage, as EEG use is rare in clinical settings and as such it can be difficult to differentiate between the two (Podell, 2012; Meland and Carrera-Justiz, 2018).
- The ictus is the seizure event itself.
- The postictal stage is characterised by further behavioural abnormalities or even actual neurological deficits such as ataxia or blindness, that persist after the seizure has stopped (Podell, 2012). These deficits are most often transient, but can take several days to resolve.
Causes
The two primary causes of a seizure in veterinary patients are a primary neurological cause, or an extracranial, metabolic issue leading to a secondary encephalopathy (Thomas and Dewey, 2016). Neurological causes for seizures can include structural disease, such as hydrocephalus, neoplasia, trauma, and infectious/inflammatory, degenerative or vascular pathologies (Podell, 2012; Thomas and Dewey, 2016). Metabolic causes for seizures consist of liver disease, electrolyte imbalances such as hypoglycaemia, polycythaemia and toxin ingestion. Porto-systemic shunt patients commonly present with seizures because of a subsequent hepatic encephalopathy, and thiamine deficiency as a result of anorexia or insufficient dietary intake is an important consideration in any cats presenting with seizure activity (Podell, 2012).
When all other potential causes for seizures have been ruled out through suitable diagnostic testing, a diagnosis of idiopathic epilepsy can be made. Many idiopathic epilepsy cases are believed to be genetic in origin, but testing for such gene mutations is currently limited and as such diagnoses of idiopathic epilepsy are believed to be overestimated in the veterinary population (Uriarte and Maestro Saiz, 2016). The International Veterinary Epilepsy Task Force (IVETF) have proposed a three-tier criteria system for having confidence in providing a diagnosis of idiopathic epilepsy, and this is summarised in Table 2 (De Risio et al, 2015).
Table 2. Summary of the International Veterinary Epilepsy Task Force three-tier criteria system for having confidence in providing a diagnosis of idiopathic epilepsy
| Confidence level/tier | Criteria |
|---|---|
| Tier I | History of two or more seizures, at least 24 hours apart Seizure onset at between 6 months and 6 years of age Normal physical and neurological examination between seizuresNo significant abnormalities on baseline blood tests and urine analysis |
| Tier II | All factors listed in Tier INormal pre- and post-prandial bile acidsNormal MRI study of the brainNormal CSF analysis |
| Tier III | All factors listed in Tiers I and IIEEG diagnosis of abnormal electrical activity characteristic for seizure disorders |
MRI = magnetic resonance imaging, CSF = cerebrospinal fluid, EEG = electroencephalogram
Diagnosis
Any patient presenting with the clinical complaint of seizures should have a full history taken and a neurological examination completed by the veterinary surgeon, ideally before any seizure medications are administered. Care should also be taken in interpreting the results of a neurological examination if a seizure has recently occurred, as post-ictal changes may create transient deficits (Podell, 2012; Meland and Carrera-Justiz, 2018). If the patient is actively seizuring on arrival, suitable drugs can be given with the understanding that subsequent lethargy and depression may make any neurolocalisation via physical examination difficult. Several other conditions can appear very similar to seizure activity, such as vestibular disease and movement disorders, making obtaining an accurate clinical history from the client imperative; if available, asking for a video of any suspected seizure activity can be invaluable (Meland and Carrera-Justiz, 2018).
A blood sample should be taken for biochemistry, haematology and electrolyte analysis in order to rule out any life-threatening metabolic causes (Saunders, 2015a). If hepatic encephalopathy is suspected, ammonia and pre- and post-prandial bile acid tests should also be conducted. Any metabolic changes may necessitate further investigations such as urine analysis or abdominal ultrasound as appropriate (Saunders, 2015a).
Once extracranial seizure causes have been excluded, attention can turn to identifying any primary neurological disease that may be present. Magnetic resonance imaging (MRI) remains the gold-standard for brain imaging because of the superior soft tissue definition and contrast but requires the patient to remain completely still in order to obtain diagnostic-quality images (Saunders, 2015a). General anaesthetic is required for all MRI scans in veterinary patients for this reason, and stabilisation of more critical patients may be required prior to undergoing anaesthesia. Any structural abnormalities will be visible on MRI, alongside any lesions, haemorrhage or pathology. Contrast administration, commonly gadolinium, enhances any pathological tissues, oedema or inflammation present in the cerebral tissues, further identifying any areas of disease in the brain (Saunders, 2015a) (Figure 1). If MRI is not available or not suitable for the patient, because of metallic implants or pacemaker, or because of financial constraints, computed tomography (CT) can be used to identify any gross cerebral abnormalities. However, it does not offer the same soft tissue definition so will miss any more subtle pathologies (Saunders, 2015a).
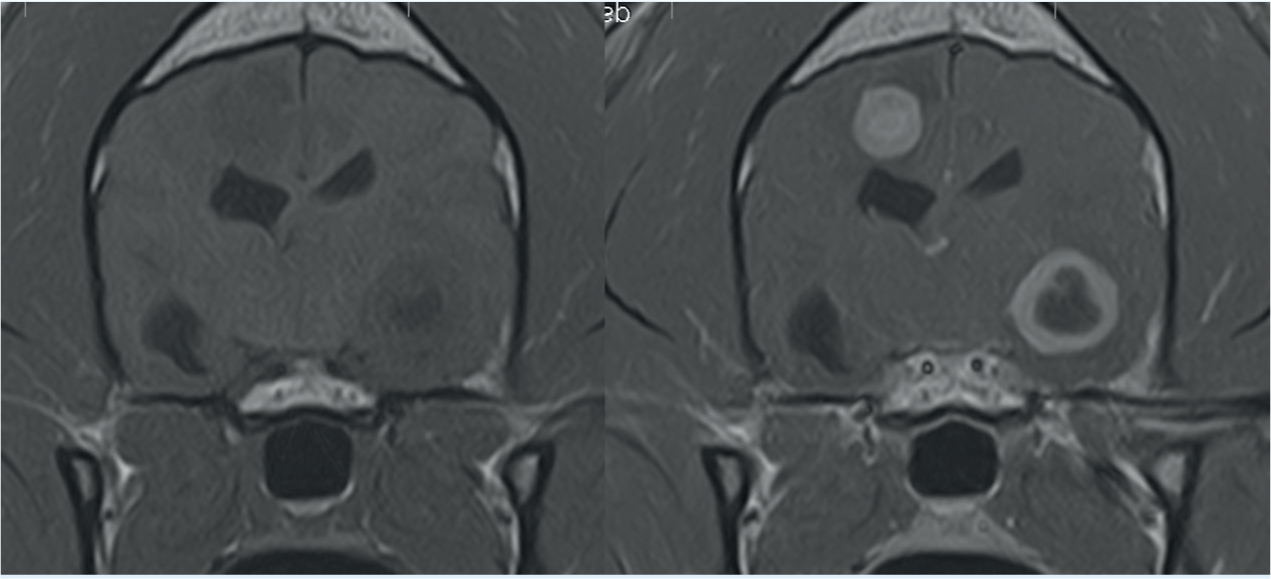
Cerebrospinal fluid (CSF) analysis obtained via the cisterna magna can assist in further diagnosing any inflammatory or infectious causes of epilepsy, many of which will demonstrate a normal MRI study (Dewey et al, 2016). Pleocytosis, an increase in white blood cells in the CSF, indicates an active inflammatory response that suggests an immunemediated disease such as meningoencephalitis (De Risio and Platt, 2014). Neoplastic conditions such as lymphoma may also be identified in CSF where an MRI study shows no abnormalities (Saunders, 2015a). Polymerase chain reaction (PCR) testing on CSF can also reveal infectious causes such as Toxoplasma gondii, Neospora caninum and feline infectious peritonitis (FIP). CSF collection is contraindicated in any cases where increased intracranial pressure (ICP) is evident on the MRI, as this may lead to secondary brain herniation, respiratory arrest and death. Other contraindications include coagulopathies or suspected vertebral fractures (Dewey et al, 2015).
In human epilepsy patients, EEG is considered the most useful modality for classifying seizure type, but is rarely used in veterinary clinical practice because of the increase in musculature on the canine skull creating recording artefacts, alongside more practical problems such as lack of equipment and patient cooperation (Uriarte and Maestro Saiz, 2016). IVETF support EEG recordings as a definitive way to diagnose idiopathic epilepsy, although recognises that it is not currently practical or recommended in veterinary practice (De Risio et al, 2015).
Treatment
If a metabolic cause has been identified, specific treatment for any abnormalities should be implemented and this may be sufficient to stop any seizure activity. Similarly, if a toxin is suspected then suitable decontamination and supportive management should result in cessation of the seizures. In patients where a primary neurological cause is found, or no cause has been identified, IVEFT have produced a framework advising on when to start anti-epileptic drugs (AEDs). IVEFT suggestions for beginning AED treatment include an interictal period of 6 months or less, evidence of cluster seizures, severe postictal signs or evidence of progression in seizure frequency or severity (Bhatti et al, 2015).
The aim of AED treatment is to provide the patient with an improved quality of life, generally measured with a reduction in the number of seizures (Saunders, 2015b; Podell et al, 2016). Prior to starting AEDs, it is paramount that a realistic conversation is had with the client so that they understand the expected outcome and the level of emotional and financial commitment required (Sines, 2018). The importance of medication timings long-term, with no missed doses or sudden cessation of treatment, should be discussed (Sines, 2018). For some clients only a seizure-free status may be acceptable, and it must be emphasised that this is not always possible (Wessman et al, 2011). Ongoing financial considerations such as drug costs, blood serum monitoring of response to treatment and complications such as refractory seizures should also be discussed (Podell et al, 2016).
A list of AEDs used in veterinary patients is summarised in Table 3. Benzodiazepines, such as diazepam and midazolam, are commonly used for the emergency management of seizures because of their rapid onset of action, but have a short duration of action and are unsuitable for long-term seizure management (Meland and Carrera-Justiz, 2018). Selection of AEDs is by the veterinary surgeon and determined by individual patient and client circumstances; a discussion of this is outside the remit of this article, but it is useful for the RVN to be familiar with the different medications and any adverse effects. It should be indicated to the client that some of the expected side effects improve or resolve within a few weeks of initiating treatment.
Table 3. Common anti-epileptic drugs
| AED | Routes of administration | Suitable for long-term treatment? | Possible adverse effects |
|---|---|---|---|
| Diazepam | IV, PR | No; short duration of action | Sedation, ataxia, respiratory depression, hepatotoxicity (cats particularly) |
| Midazolam | IV, IM, IN | No; short duration of action | Sedation, ataxia, respiratory depression |
| Phenobarbital | IV, PO | Yes | Sedation, ataxia, respiratory depression, polydipsia, polyuria, polyphagia, hepatotoxicity, dermatological disease |
| Levetiracetam | IV, PO | Yes | Sedation, respiratory depression, polycythaemia |
| Potassium Bromide | PO | Yes | Sedation, ataxia, polydipsia, polyuria, polyphagia, pancreatitis, vomiting, diarrhoea |
| Imepitoin | PO | Yes | Polyphagia |
| Zonisamide | PO | Yes | Sedation, ataxia, vomiting, anorexia, hypersensitivity reaction |
IV = intravenous, PO = per os, IM = intramuscular, IN = intranasal, PR: = per rectum
Vagal nerve stimulation, dietary alterations and acupuncture have all been suggested as alternative treatments to AEDs because of their success in the management of human epilepsy, but scientific evidence does not currently support their use in veterinary patients (Bhatti et al, 2015; Uriarte and Maestro Saiz, 2016).
Nursing care
The arrival to the veterinary practice of an actively seizuring patient can be distressing, and a swift triage and administration of treatment under veterinary direction alongside management of client expectations are all roles the RVN can perform. Any seizuring patient should be handled in a calm manner to avoid further stimulation that might prolong seizure activity. Taking the patient away from the owner, or allocating them a quiet, dark room can assist with providing a suitable environment. The patient should be placed on a soft surface and any furniture should be moved away so as to avoid self-injury during the course of the seizure. No attempts should be made to immobilise the patient or secure their airway; this is more likely to result in injury to the patient or to the client/RVN. The increase in metabolic activity during a seizure subsequently increases the body's oxygen demands, so flow by oxygen should be administered if available (Thomas and Dewey, 2016). Care should be taken once the seizure has ended and the patient is coming around; post-ictal behavioural changes can include unexpected aggression (Podell, 2012).
If the VS is available to instruct, administration of an AED may be considered. Intravenous (IV) access can be difficult during a seizure, and so rectal diazepam or intranasal midazolam are often preferred (Charalambous et al, 2017). Special adaptors are available to administer drugs intranasally, or alternatively a small syringe works well. It is sensible to place an IV catheter as soon as possible after a seizure has finished, to secure a route for the administration of further AEDs should additional seizure activity occur. The use of an extension line can be considered for patients known to have tonic-clonic seizure activity to make the administration of AEDs possible without restraint of the patient's limb mid-seizure.
A full clinical examination should be conducted as soon as is reasonable for all seizure patients, alongside a spot glucose check to rule out life-threatening hypoglycaemia that would require immediate treatment. Hyperthermia is a common post-ictal finding because of the increased muscle activity, and active cooling should be initiated if the patient has a rectal temperature above 40°C (Sines, 2018). Rebound hypothermia may occur, especially if the patient is heavily sedated post-seizure, so careful monitoring of any cooling methods is necessary. Cessation of active cooling is recommended when the patient temperature is below 39°C, with frequent temperature checks continued to ensure subsequent warming is not required (Scislowicz, 2015). Further treatments, such as lactulose enemas, may also be required if a primary metabolic cause is identified (Saunders, 2015a).
Hospitalisation
Many seizure patients will require subsequent hospitalisation for monitoring and further diagnostic tests, and choice of kennel should be considered. The kennel should be in an easily observed and accessible area of the practice, but ideally in a quiet environment to avoid overstimulation that may lead to further seizure activity. It should also be large enough that they are unlikely to injure themselves should they seizure. Additionally, many seizuring patients will behave normally between seizures, and being kennelled in itself will lead to anxiety and stress that could precipitate seizure activity. In these patients a light sedation may be considered, under veterinary direction.
The use of a seizure plan (Figure 2) can be invaluable in a busy veterinary practice, as it allows the swift administration of AEDs in the event of a seizure even if the veterinary surgeon is not present (Hall and De Risio, 2010). A seizure plan is a predetermined drug and dose with a precalculated volume, decided by the veterinary surgeon on admission of the patient, that is then displayed on the patient's kennel so that any RVN that identifies a seizure is able to immediately administer the required medication in a timely manner. The plan may also specify a protocol for the occasion that the patient continues seizuring despite AED administration; this may be repeat doses or a different medication. The desired drugs should also be easily accessible near to the patient, with prepared needles, syringes and saline flush all available to make administration in a seizure as quick as possible (Hall and De Risio, 2010).

Intracranial pressure
The skull is considered a closed cavity with a finite volume and is occupied by the brain itself alongside blood and CSF. A careful homeostatic balance is maintained by the body to ensure that the pressure within the skull is kept within careful limits in order to prevent damage to cerebral tissues (Raisis and Brearley, 2012). Any increase in intracranial pressure (ICP) is dangerous, as reduction in cerebral perfusion alongside compression of the brain tissue itself can lead to irreversible cerebral hypoxia and subsequent brain herniation and death (Raisis and Brearley, 2012). Seizure patients should be closely monitored for any signs of increased ICP, as many differential diagnoses for seizures alongside seizure activity itself can lead to inflammation and oedema, subsequently leading to increased pressure inside the skull.
There is no direct way to monitor ICP in veterinary patients, but frequent monitoring for any deterioration in the patient's demeanour or neurological status can indicate a problem. The Cushing reflex is a cardiovascular response to increased ICP and can be an extremely useful monitoring tool (Saunders, 2015c). An increase in ICP causes compression of cerebral vasculature, necessitating an increase in systemic blood pressure in order to maintain cerebral perfusion and prevent cerebral hypoxia. This systemic hypertension is recognised by baroreceptors in the carotid arteries, which generate a reflex bradycardia. Any seizure patient presenting with hypertension, systolic blood pressure above 180 mmHg, and bradycardia, heart rate below 40 beats per minute, should be flagged to the veterinary surgeon for immediate treatment.
Treatment of increased ICP is via diuretics, most commonly mannitol or hypertonic saline (Saunders, 2015c). These act by drawing fluid from the interstitial and intracellular spaces into the intravascular space, improving cerebral blood pressure and perfusion while subsequently decreasing ICP (Raisis and Brearley, 2012). Both are administered as boluses over 10–20 minutes, but a filtered infusion set must be used for mannitol to prevent accidental administration of crystals. Suitable isotonic fluid therapy is recommended after the use of a diuretic to prevent dehydration (Saunders, 2015). Prevention of ICP increases is another consideration for any seizure patient, even if they appear clinically well. Occlusion of the jugular vein should be avoided, with any blood samples taken from peripheral veins. Coughing and emesis should be avoided, which is a consideration when selecting drugs for anaesthesia or intubating the patient. Harnesses are preferred to neck leads, and head elevation to between 15 and 30° is recommended in recumbent patients (Saunders, 2015c).
Status epilepticus
Any patient that presents with seizure activity lasting longer than 5 minutes, or that does not recover fully between seizure episodes, is defined as being in status epilepticus (Mariani, 2013; Thomas and Dewey, 2016). This is considered a medical emergency, and immediate action must be taken to prevent cellular damage in the brain (Thomas and Dewey, 2016; Sines, 2018). Continuous seizure activity causes inflammation and oedema in cerebral tissues, disrupting the blood–brain barrier and predisposing to further seizure activity (Librizzi et al, 2012). In addition, the increased metabolic demands on the body eventually overwhelm the body's compensatory mechanisms, leading to hypoglycaemia, hypoxia and a lactic acidosis, with subsequent death if the seizure activity is not controlled (Scislowicz, 2015).
If not responsive to initial AEDs, a constant rate infusion (CRI) may be considered to halt the seizure activity while more long-term medications are administered. Midazolam, diazepam and propofol are the most commonly used, with the aim being to halt the seizure activity at the lowest possible dose. The use of propofol CRIs in cats is not recommended because of the risk of Heinz body anaemia, but if used monitoring of the patient's haematocrit is highly recommended (Podell, 2012). Use of a syringe driver is paramount to ensure accurate dosing and sterility must be maintained when handling all intravenous medications. CRIs are usually continued for 6–12 hours while patients are loaded with additional AED medication, before slowly being decreased with close monitoring for refractory seizure activity (Podell, 2012; Scislowicz, 2015).
Nursing care of any patient on an AED CRI will depend on the level of sedation required to halt the seizure activity. Airway maintenance is paramount, and if high doses of propofol are required then endotracheal intubation may be required to secure the airway (Scislowicz, 2015). Oxygen requirements will be increased because of the high metabolic demands, and oxygen supplementation by the most suitable method is highly recommended (Thomas and Dewey, 2016). Recumbency care, bladder management and fluid therapy are all important considerations in these patients, alongside ocular lubrication and frequent monitoring of vital parameters. While hyperthermia is common in the actively seizuring patients, once on a CRI they may quickly become hypothermic and so active warming may be indicated (Scislowicz, 2015). Heart rate, respiratory rate and pattern, blood pressure and oxygen saturation should all be regularly monitored and recorded alongside any suspected breakthrough seizure activity and medications administered (Hall and De Risio, 2010).
Conclusion
Nursing the seizure patient can be a frustrating experience, especially where refractory seizure activity is present. However, it can be extremely rewarding to see these patients recover and achieve a good quality of life, something in which the RVN is well poised to play a vital role. While AED therapy and management is primarily down to the VS, by maintaining a holistic nursing approach the RVN is able to tailor the experience for the individual patient and assist the client in making informed decisions, ultimately providing the most suitable outcome for all parties involved.
KEY POINTS
- Seizures are a common presenting problem in companion animals that occur as a result of excessive firing of neurons in the brain.
- There are numerous different types of seizure, as well as other disease processes that can mimic seizure activity, and it is important for the veterinary team to be able to distinguish and accurately describe the difference between them.
- Idiopathic epilepsy is a diagnosis of exclusion, typically used to describe a patient that seizures multiple times throughout their life without any evidence of structural or metabolic disease.
- Seizures can be upsetting for owners to witness, and the veterinary nurse is well placed to support clients and coach them through subsequent management options.
- Intracranial pressure is an important consideration in all seizure patients, with careful monitoring to prevent further complications such as brain herniation.
- Constant seizure activity for longer than 5 minutes is defined as status epilepticus, and requires immediate treatment to prevent cell death throughout the body.


