Tick-borne encephalitis virus (TBEV) is a flavivirus closely related to, but distinct from louping ill virus (LIV) in sheep. Although TBEV is known to be less virulent than LIV in sheep (Gritsun et al, 2003), it can cause a neurological disease known as tick-borne encephalitis (TBE) in humans, and less commonly, dogs. There are three subtypes of TBEV currently recognised (European, Siberian, and Far Eastern), although Baikalian and Himalayan subtypes have also been proposed (Dai et al, 2018).
The European subtype has rapidly spread across Central and Western Europe in recent years, where it is primarily transmitted by Ixodes ricinus ticks and maintained in endemic foci. In endemic areas, the prevalence of TBEV in questing ticks rarely exceeds 1%, even where human incidence of disease is high (Imhoff et al, 2015). The risk of human and canine infection from short visits to endemic areas with limited tick exposure is therefore low. People and pets living, frequently visiting or working in endemic areas, however, would be at significantly greater risk of exposure over time. This has made the possibility of TBEV establishing in the UK in the face of an increasing European distribution, increased pet travel and pet importation a concern, as endemic domestic foci would put the UK human and canine population at greater risk. That concern now appears to be a reality, since evidence has emerged that endemic foci are present in the UK, particularly in the Thetford Forest.
Prevalence of TBEV in Europe and presence in the UK
The distribution of TBEV is closely related to the activity of its tick vectors. These are Ixodes ricinus (Figure 1) in Western and Central Europe and Ixodes persulcatus in Central and Eastern Europe. Rodents act as reservoir hosts capable of maintaining the pathogen once it has moved to a new area. Larger wild animals such as deer are not considered to be competent hosts for virus transmission but serve as transport hosts for infected ticks, allowing maintenance of tick populations and geographical spread of TBEV. Migratory birds are also likely to play a significant role in carrying infected ticks over large distances.

The European virus subtype has spread rapidly and is endemic in Scandinavia, Western and Central Europe, and countries that made up the former Soviet Union. Human TBE is common in the Baltic states, Austria, Germany, Hungary, Poland, Switzerland, Russia and the Ukraine. It has also been reported at a lower frequency in Bulgaria, Romania, Denmark, Sweden, Finland and France. Sporadic cases have also been reported from Albania, Greece, northern Italy, Norway, and Turkey.
The spread of TBEV in Western Europe was confirmed when the Netherlands reported its first human case in 2016 (Jahfari et al, 2017).
A surveillance programme carried out by Public Health England in 2018 looked for evidence of TBEV in wild animals and ticks. Serum was collected from 1309 deer culled across England and Scotland. Of these samples, 4% were ELISA-positive for TBEV, with foci in the New Forest and Thetford Forest. The Thetford Forest area had the highest proportion (47.7%) of seropositive samples. Engorged ticks collected from culled deer within seropositive regions were tested for viral RNA, with 5 of 2041 ticks testing positive by LIV/TBEV real-time reverse transcription PCR. All of the positive ticks were from the Thetford Forest area, with a full-length genomic sequence of TBEV being identified in one tick (Holding et al, 2020). This is strong evidence for TBEV being present in at least one endemic focus in the UK, and possibly more.
Clinical presentation
The incubation period in humans before clinical signs occur is 3–28 days, producing clinical disease in an estimated one third of TBEV infections. The disease typically has a biphasic course, starting with flu-like symptoms, followed by a symptom-free interval before neurological disease occurs. The severity of the neurological phase can vary from mild meningitis to severe encephalitis with or without myelitis and spinal paralysis (Lindquist and Vapalahti, 2008). Between 1% and 5% of cases are fatal. The disease caused by far eastern TBEV does not typically follow a biphasic pattern but presents with massive headache, high fever and vomiting. Delirium, coma, paralysis, and death may follow, with a fatality rate of 25–30%.
Seropositivity for TBEV has been found in many domesticated animals including dogs, but TBE in dogs appears to be relatively rare compared to in humans. In endemic areas in Switzerland there is a higher seroprevalence in dogs than humans, yet the incidence of clinical cases in dogs in Switzerland, Austria, Germany and Sweden is significantly lower than the human incidence of disease. Seropositive dogs investigated in Denmark in 2005 and 2006 found no evidence of TBE. In a serological survey performed in dogs from Sweden in 1992, however, 16 of 18 seropositive dogs showed neurological signs including ataxia, atonic paresis and sensitivity to sound (Bjöersdorff, 2002).
The potential for dogs to develop clinical signs means that TBE should be considered in dogs with central nervous system neurological signs that have visited or lived in endemic countries. Dogs at risk of tick exposure in these countries should also have adequate tick prevention. The incubation period for canine TBE is typically between 7–14 days and is febrile in nature with a variety of neurological signs including ataxia, proprioceptive deficits, seizures, tremor, paresis, paralysis and cranial nerve deficits such as facial paresis. Neurological signs are often progressive and can be fatal.
Positive dogs pose no significant risk to owners, as ticks are required for transmission. They are, however, sentinels for human infection, as dog owners are likely to be at risk of infected tick exposure themselves from walking in the same areas as their dog.
Diagnosis and treatment
Diagnosis of TBE is difficult in both humans and dogs. A definitive diagnosis relies on isolation of the causative virus from blood or cerebrospinal fluid but carries a poor sensitivity. Negative results therefore do not rule out infection. IgG serology can be used with a fourfold increase in titres indicative of acute infection. However, there is cross reactivity with other flaviviruses and this possibility needs to be considered in the event of a positive result.
There is no specific chemotherapeutic agent that can target the virus itself, treatment therefore is supportive and symptomatic. Pain relief and intravenous fluid therapy may be required alongside anticonvulsives, sedatives and muscle relaxants as required (Pfeffer and Dobler, 2011). Non-steroidal anti-inflammatory drugs may be needed for pyrexic dogs and antibiotics for secondary bacterial infections such as pneumonia. Dogs that survive TBE often require 6–12 months to make a full recovery.
Prevention of disease
The potential severity of TBE makes promoting awareness and prevention of the disease vital for dogs and owners whose lifestyle may put them at increased risk in endemic areas. This includes those who spend time walking in rural areas, farmland, bracken and deer-inhabited woodland. Dogs travelling to endemic countries should also be protected. The establishment of TBEV in the UK may cause concern among clients who live or exercise their pets in endemic areas. Veterinary professionals should keep the relatively low risk of exposure in perspective for clients, while encouraging preventative steps to keep risks to dogs to a minimum. Preventative measures consist of:
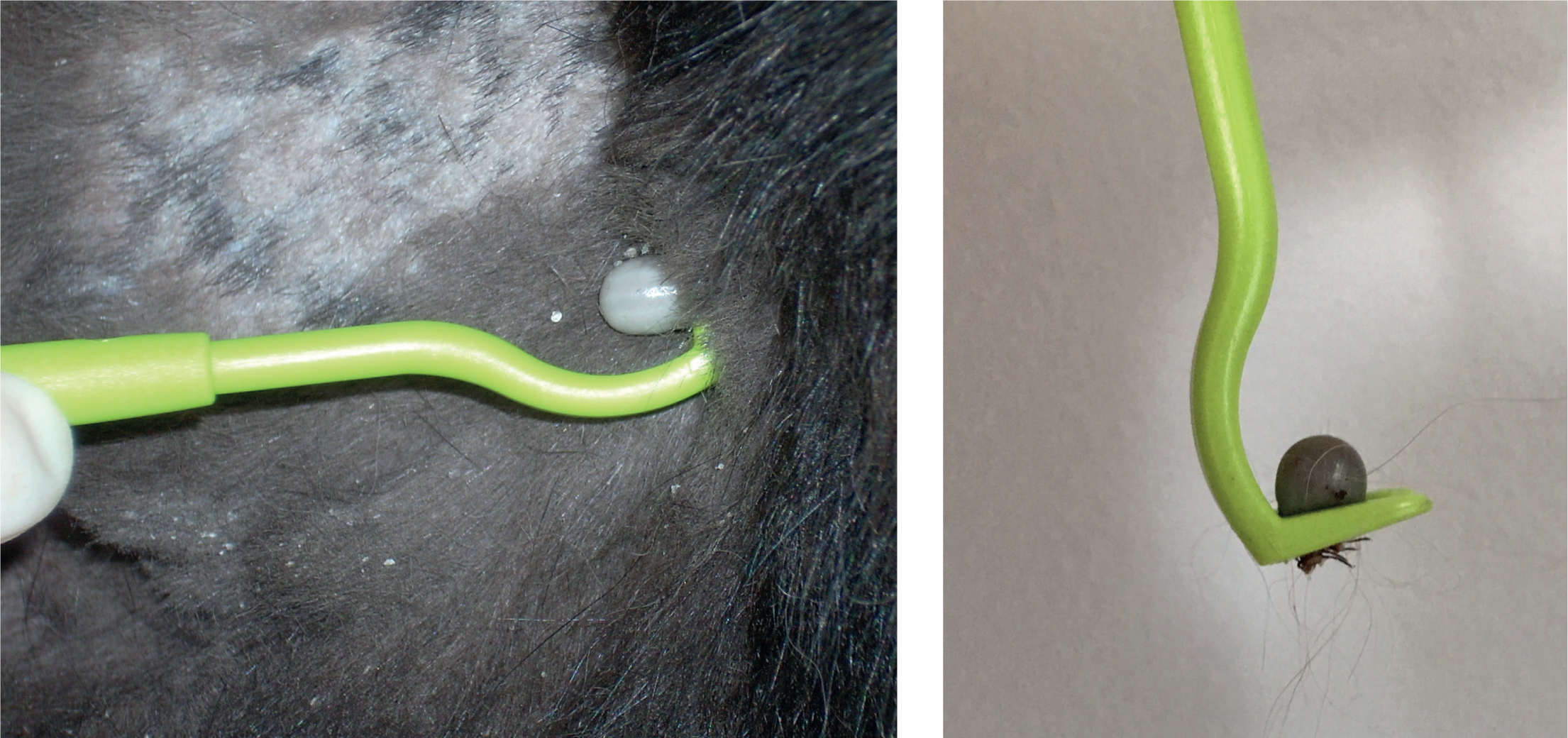
- Daily monitoring for ticks. Clients should be advised to check their pets every 24 hours or after outdoor activity in endemic areas. Any ticks found should be carefully removed with a tick hook (Figure 2a and b), using a simple ‘twist and pull’ action. It is important that owners are instructed how to remove ticks without stressing them and without leaving the mouthparts in situ. Removal can also be performed with tweezers, but they should be fine-pointed and not blunt, as crushing will stress the tick, causing it to release stomach and salivary gland contents, increasing the risk of disease transmission as a result. Traditional techniques to loosen the tick, such as the application of petroleum jellies or burning, will also increase this likelihood and are contraindicated. When checking for ticks, clients should be reminded that nymphs are very small and may easily be missed, especially in areas of long fur. If examination is difficult or time limited, then the majority of ticks are found around the head, neck and limbs, therefore the search should concentrate on these areas (Wright et al, 2018).
- Use of tick preventative products. The use of prophylactic compounds that rapidly kill or repel ticks is useful in reducing tick feeding and therefore transmission of infection. Products containing an isoxazoline (e.g. Bravecto® (MSD), Credelio® (Elanco), Nexgard® (Boehringer Ingelheim Animal Health UK Ltd), Simparica® (Zoetis)), permethrin (Activyl plus® (Virbac), Advantix® (Bayer) and Vectra 3D® (Ceva) spot on solutions), deltamethrin (Scalibor® collar (MSD)) and flumethrin (Seresto® collar (Bayer)) all fulfil these criteria. It is important to consider compliance when discussing which product to use, as well as lifestyle. Whether a client prefers or is able to administer a tablet, collar or spot on should be established, as well as whether the pet has had reactions to products in the past. Additionally, frequent swimming or bathing may make some topical products unsuitable. It should also be remembered that no product is 100% effective, and owners should therefore still be advised to check their pet for ticks at least every 24 hours if possible.
Infected Ixodes ricinus ticks on imported and travelled animals may introduce the virus into new areas, with the potential for further endemic foci to establish. Pets arriving in the UK from abroad should therefore be checked for ticks and if not already treated, a tick treatment applied.
In Europe, there are currently two vaccines that are licensed for human use, but neither have been licensed for animal use, including dogs. Off-licence studies in dogs have shown some efficacy, but further safety and efficacy studies would be needed before they could be routinely used in high risk areas (Pfeffer and Dobler, 2011).
Conclusions
Tick-borne encephalitis virus is the most significant tickborne virus affecting humans and dogs in Europe and a potentially fatal zoonosis. Its rapid spread across Europe and establishment is therefore of concern and another compelling reason for tick preventative strategies in dogs at high risk of tick exposure. Veterinary professionals have a vital role in keeping the risks of exposure in perspective for pet owners, while also ensuring that adequate prevention is in place for their pets. Vigilance in imported and travelled pets is also an important component of preventing or slowing future spread.
KEY POINTS
- Tick-borne encephalitis virus is a significant and potentially fatal viral pathogen of dogs and humans.
- The virus has spread rapidly across Europe and now appears to have established in the UK, putting domestic and travelling dogs at increased risk of exposure.
- Dogs do not pose a direct risk to humans, but are useful sentinels of infection.
- Dogs are not as susceptible to disease caused by the virus as humans are, but it can still be fatal in affected dogs.
- Adequate tick prevention in dogs is therefore vital both to keep individual dogs safe and to help limit further geographical spread.


