Despite the advantages of modern molecular approaches to parasitic disease diagnosis, the coprological methodologies still seem to be the most widely used methods (Ward et al, 1997; Cringoli et al, 2010; Vadlejch et al, 2011). This is due to their being inexpensive, fast and logistically feasible for the ordinary veterinary clinic or veterinary hospital, using low complexity materials and reagents. Thus, classical coprological methods allow for quick and reliable detection of parasites. However, the detection of these parasitic elements may be insufficient and further data might be required to allow definitive diagnosis, specifically the quantification of parasitic forms. As such, faecal egg counts can play a crucial role in monitoring helminth loads, determining the extent of pasture contamination, allowing also anthelmintic resistance identification and sustaining epidemiological studies (Nichols and Obendorf, 1994; Ward et al, 1997; Vadlejch et al, 2011). There are a number of faecal egg count methods that quantify the number of parasitic elements per weight of faeces, and all of them are based on the microscopic examination of a stool suspension. Among all faecal egg count methods described, the McMaster method stands out for being the most widely used in veterinary parasitology. In fact, the World Association for the Advancement of Veterinary Parasitology (WAAVP) recommends the use of the McMaster technique for both the screening for anthelmintic resistance (Coles et al, 1992) and testing anthelmintic drug efficacy in ruminants (Wood et al, 1995).
The McMaster technique was developed at the McMaster laboratory of the University of Sydney and first published in 1939 in the Journal of the Council for Scientific and Industrial Research by HM Gordon (an officer of the Council´s McMaster Animal Health Laboratory, Sydney) and HV Whitlock (a laboratory assistant of the McMaster Animal Health Laboratory, Sydney) (Gordon and Whitlock, 1939). In this paper, the authors pointed out several disadvantages of the former technique, namely the time used for sample preparation and the presence of debris obscuring eggs, which hampered the microscopic observation. With the new technique these problems would be surpassed, by the introduction of what they called ‘the special slide’. This was the first draft of what is known today as the McMaster counting slide.
Since its first description in 1939, many variations of the McMaster technique have been reported for use in both animal and human diagnostics, with method modifications using different types of flotation solutions and faecal weights, flotation times, sample dilutions, additional centrifugations for clarification of suspensions (with different centrifugation times and speeds), numbers of McMaster slide sections counted for calculation purposes and different interpretation coeficients (Cringoli et al, 2004; Pereckiene et al, 2007; Karamon et al, 2008; Vadlejch et al, 2011).
In short, and despite its variations, the traditional McMaster parasitologic technique uses a counting chamber (Figure 1) which enables a known volume of faecal suspension (2 x 0.15 ml) to be examined microscopically. Thus, if a known weight of faeces and a known volume of flotation fluid are used to prepare the suspension, then the number of eggs per gram of faeces (epg) can be calculated.

The McMaster method
Equipment needed:
Step-by-step guide






How to obtain, register and interpret results
Register the results of the test in the laboratory log book so as to avoid missing documentation in the future. Whenever possible record the identification and the counting results.
The McMaster chamber has (normally) two compartments, each with a grid etched onto the upper surface. When filled with a suspension of faeces in flotation fluid, much of the debris will sink while eggs float to the surface, where they can easily be seen and those under the grid counted.
The quantities are chosen so that the faecal egg count can be easily derived by multiplying the number of eggs under the marked areas by a simple conversion factor.
The number of eggs per g can be calculated as follows:
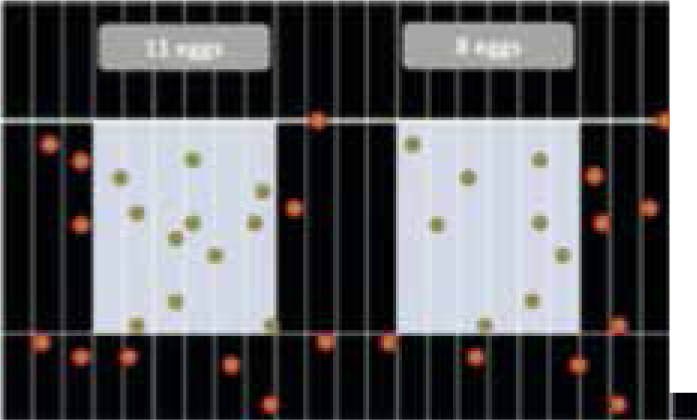
For example (Figure 8): according to the example there are (11 + 8) x 50 = 950 epg.
In a three compartment chamber, with 11 eggs in first compartment, 8 in the second and 10 in the third (Figure 9). The number of eggs per gram can be calculated as follows:
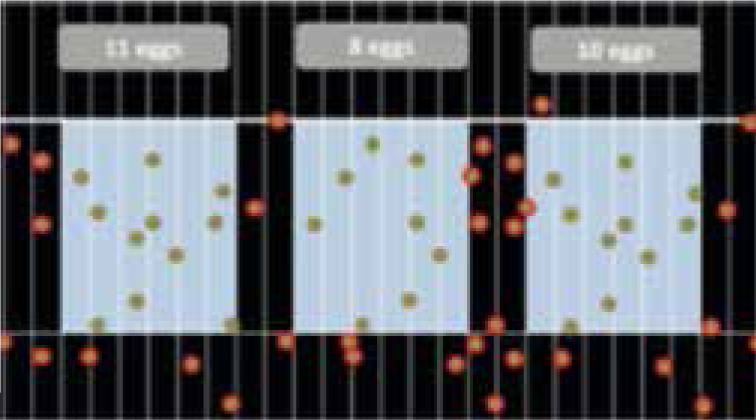
According to the example there are (11 + 8 + 10) x 33.33 = 966.57 = 967 epg.
Note: It is very important to not delay the count beyond the recommended time, since there are fragile eggs that can be destroyed or distorted by the flotation fluid.
Further considerations
To end this second article on ABC parasitologic series, it is important to highlight the biological hazards associated with handling stools. As a rule of thumb, one should always consider that faeces and faecally contaminated surfaces and equipment are potentially infectious and thus hazardous to those performing sample collection and laboratory diagnostics. As such, it is important to wear personal protective equipment including protective outerwear and disposable gloves when handling stools and stool contaminated materials. General, simple and good personal hygiene measures must be ensured such as washing hands before touching clean items and avoiding eating and drinking on the premises, even when no diagnostic procedures are taking place. Additionally, potentially contaminated waste must follow the hospital's procedure for waste disposal. In general, stools should be disposed of in bags or containers labelled with a biohazard symbol, however, if sharps are contaminated with biohazardous material they should be disposed of in the contaminated sharps container.
Conclusion
Parasite identification, although of value for diagnosis, is often insufficient and additional quantification is required for definite conclusions. The authors recomend the McMaster protocol, widely known as the most used faecal egg count method.

