The Baermann technique is a simple, low-cost diagnostic tool for diagnosis of parasitic larval forms, that parasitologists, health and veterinary personnel rely on. Historically, this technique is derived from attempts to identify nematodes from the soil, and relies on the fact that these parasites are aquatic animals and that almost all are mobile (Webster et al, 2008). In 1917, while working in Java, the Dutch physician Baermann devised a simple method of isolating nematodes, including infective hookworm larvae, from soil. It became known as the Baermann funnel technique, or Baermann technique (Baermann, 1917). Dr Baermann discovered, by putting soil in a muslin bag and into a funnel filled with water for several hours, that nematodes tended to migrate downwards out of the soil and through the muslin, and then could be seen in the water at the stemend of the funnel. Despite the fact that this was ground-breaking, Baermann´s discovery presented a drawback, with the water resulting from this method being often murky (as a result of the leaching of pigments and particles of small diameter through the muslin) (Webster et al, 2008). This has led to several modifications in the methodology.
Following its application to human and veterinary parasitology, it has been shown that the Baermann technique has advantages over other tests used for the same purpose (Bowman et al, 2002). In particular, since larvae may be present in low numbers or intermittently, the Baermann technique allows for the use of a larger amount of stools to be used compared with a faecal flotation test (Bowman, 2009).
Despite the fact that the Baermann technique is possibly the easiest morphology-based test to perform and evaluate in diagnostic parasitology, it still seems to be rarely used in veterinary practice. It is worth pointing out some of the difficulties that exist for the execution of the technique, such as the size of the equipment, space required in the lab and the large volumes of faeces required. Additionally, there is the need for experience when identifying specific parasitic larvae and therefore for increased specificity of the test. Nonetheless, it presents great value and potential for veterinary use in the extraction of live larval stages of nematode parasites from faeces (Zajac and Conboy, 2012).
The Baermann technique is based on the active migration or movement of larvae. In brief, faeces are suspended in water and the larvae move into the water. Since they are unable to swim, they sink to the bottom and can be collected for identification.
In the Baermann test it is essential to use a fresh faecal sample, collected immediately after passage from the animal, since the contact with the ground can contaminate faeces with freeliving nematodes that will also be recovered in the test and that can be hard to distinguish from parasite larvae. Also it is not recommended to refrigerate samples for a lengthy period (days), since larvae may die in that time, and the test requires the presence of live larvae, able to react to a stimulus such as the warm water used in this test (Bowman, 2009).
Herewith we describe the Baermann technique:
The Baermann technique
Equipment needed:
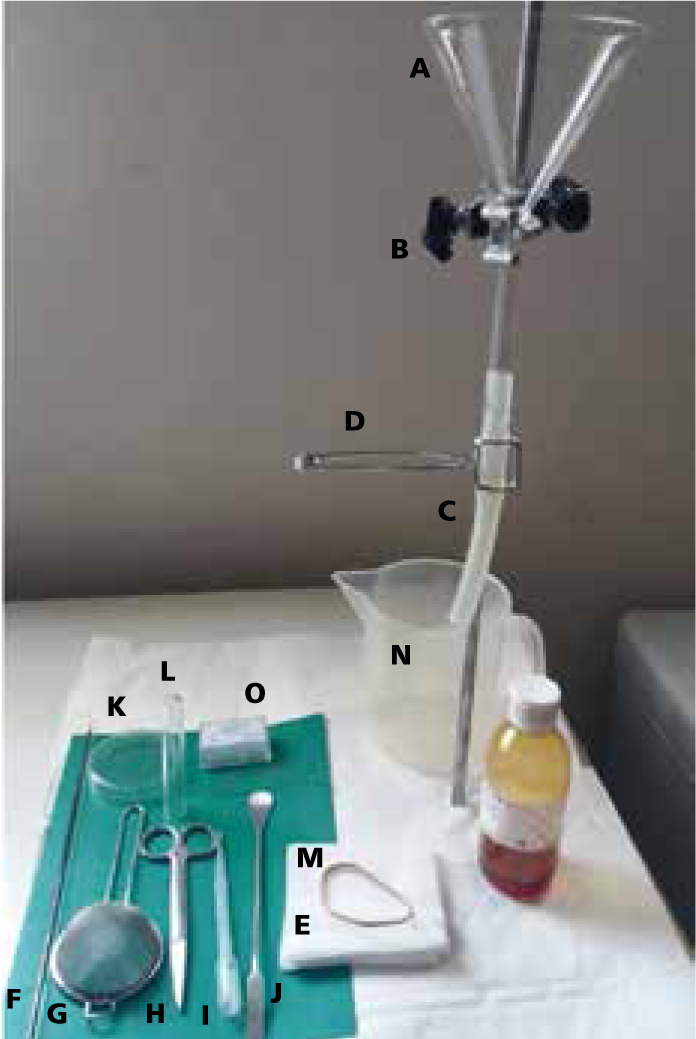
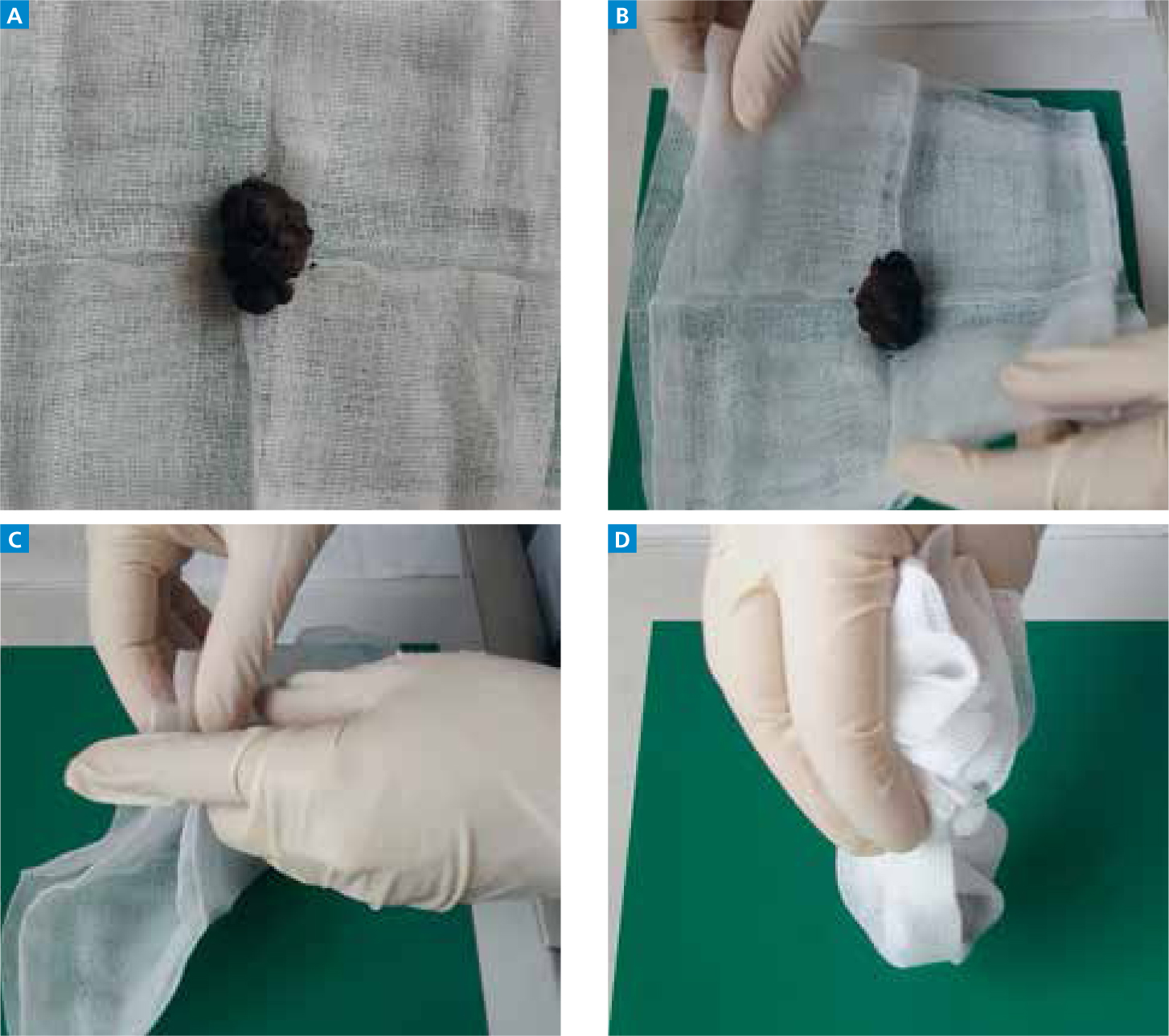
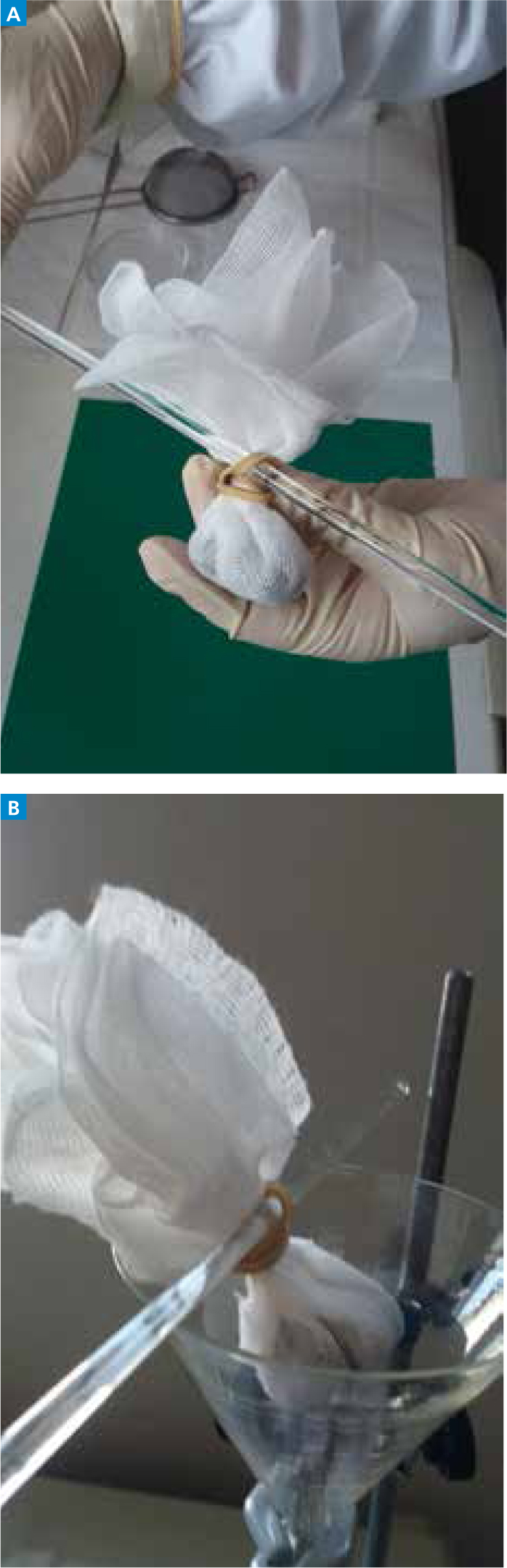
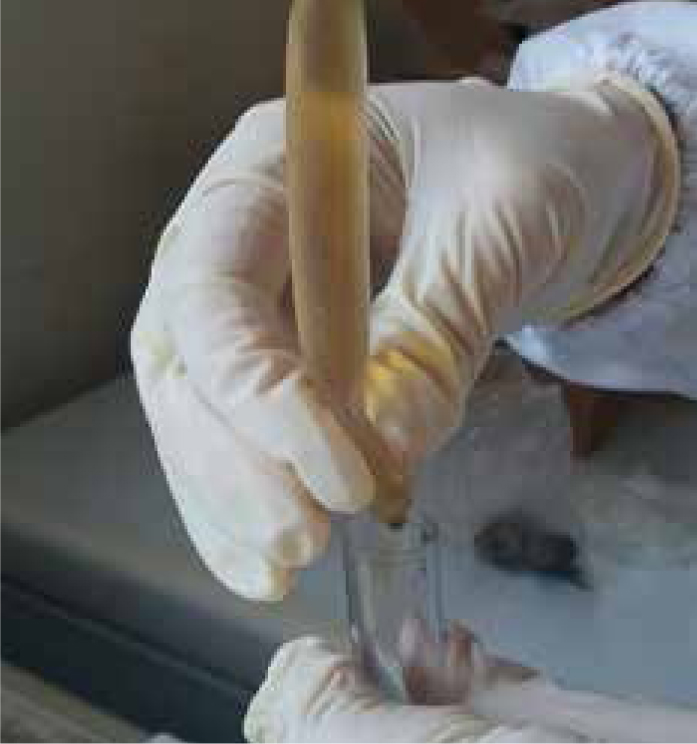
Step-by-step guide
Note: Free-living nematodes stain deeply brown in iodine and can be distinguished from parasitic species by the presence of a double bulbed (rhabditiform) oesophagus.





Further considerations
As with other diagnostic procedures in parasitology, it is important to highlight that stools are biological materials that carry risk of human infection, namely for the person carrying out the technique. As such and for the safety of the user, faecal material should always be presumed to be infectious and protective outerwear and disposable gloves should be used at all times. General health and safety regulations for biological material manipulations should always be followed.
Conclusion
The Baermann technique is not recommended as a primary diagnostic technique for evaluation of parasites in faeces, but is very useful to detect nematode larvae (L3). It is easily executed, however it requires experience to identify parasitic species present.

