Lameness is one of the most common issues seen in equine practice, historically complex to diagnose, many practitioners and owners can be frustrated with the ever-eluding causation of lameness. Traditional methods of imaging can be insensitive to subtle changes in the equine patient which can cause significant discomfort. Routine nerve-blocking with local-anaesthetic and lameness assessments alone can be timely, costly, and a painstaking process, which can often leave a lot unanswered. However, over the last decade magnetic resonance imaging (MRI) has become more accessible and commonly utilised in veterinary diagnostics (Smith, 2015a; Mizobe et al, 2016). Having been the case for years within human medicine, specialist facilities and public perception have aligned to enable practitioners the scope to advance their field and, in turn, provide outstanding quality images and accurate diagnoses; promoting animal welfare (Swagemakers et al, 2016). With the ability to depict bone, tendon, ligament, and other soft tissue lesions including those within the hoof capsule, MRI supersedes the accuracy and detail capability of many other imaging techniques (Dyson et al, 2003) (Figure 1). Within the UK the majority of equine MRI scanners are low-field standing units, with only three high-field general anaesthetic units in the country. Both modalities have opposing merit, however, the field of interest, cost and the holistic presentation of the patient play a vital role in making informed choice between the two (Biggi and Dyson, 2018). Although considered safer, there are still some patient considerations to be addressed when using the lowfield scanners; long periods of sedation and a lack of food and water can lead to a higher probability of gut stasis and impaction (Bailey et al, 2016). Low-field scanners are often limited to the distal limb, due to the structure of the machine and movement artefacts associated with standing sedation. Although movement is alleviated via general anaesthetic in high-field, high-field scanners also have a better capacity to image the proximal limb structures, head, and cervical vertebrae of the horse. However, this is accompanied by the risk of general anaesthesia, which is widely known in horses to carry a high morbidity and mortality risk (Johnston et al, 1995; Senior, 2013).
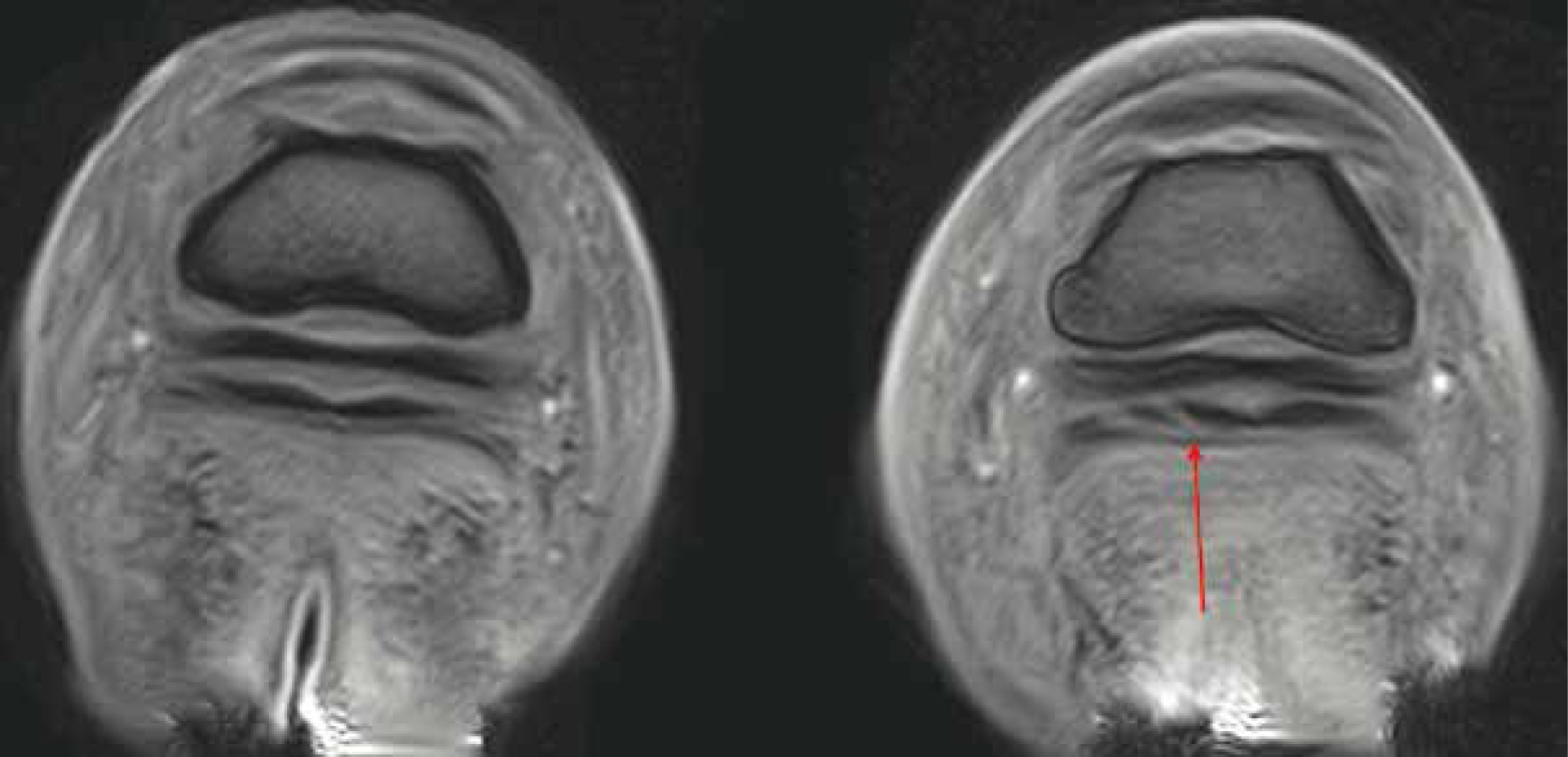
MRI
Unlike many other imaging tools such as radiography and computed tomography, MRI does not use ionising radiation and therefore holds no known biological hazards. Although researched extensively, none have confirmed a negative association with the use of a static magnetic field (Bolas, 2010). MRI uses a combination of radio waves, strong magnetic fields, and computer technology to depict detailed images of internal structures. Considering the composition of the body (approximately 60–70% water) MRI uses the vast amount of hydrogen nuclei (protons), which are magnetic, to create the images. Once applied, the spinning protons rotate to align with the magnetic field. A radiofrequency pulse is then applied, which forces the proton out of alignment to 90° or 180° (Reddy et al, 2012). Following this, the radiofrequency waves are removed allowing the proton to move back into its natural position with the magnetic field (Bolas, 2010). Sensors detect the time taken for the protons to return to their original alignment while emitting electromagnetic energy, which the computer converts into highly detailed black and white images of the structure (Formica and Silvestri, 2004). Unlike traditional imaging techniques, MRI has the ability to show both soft tissue and bone simultaneously in high resolution (Daniel et al, 2013; Smith, 2015a). Widely considered to be the ‘gold standard’ of diagnostic pathology, MRI allows detailed cross-sectional imaging of the target structures using multiple planes to depict up to 500 images per hoof, which can then be analysed by a specialist (Hallmarq, 2018) (Figure 2). Due to the sensitive nature of MRI, minor changes within the tissue are illustrated, enabling accurate prognosis to be determined and concise treatment, where appropriate, to be implemented (Werpy, 2010).
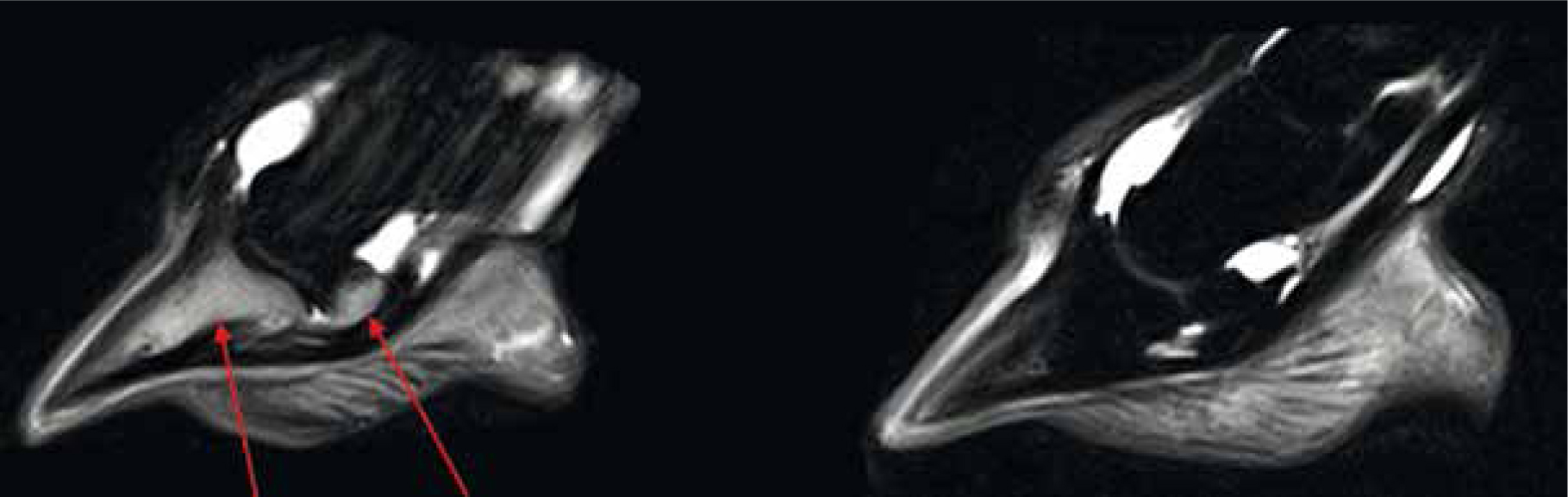
High-field MRI
Often tubular in shape, high-field MRI, otherwise known as superconducting MRI, is capable of scanning to the proximal limb, head, and cervical vertebrae; this process requires the use of liquid helium to cool the wires which aid in the creation of the magnetic field as part of the superconducting system. Turning off the superconducting magnets requires the liquid helium to be emptied from the system, which is not only expensive but also dangerous to personnel involved (Swagemakers et al, 2016). High field MRI scanners (>1 Tesla) have a much stronger magnetic capacity and therefore produce higher resolution images than the low-field systems. Previous studies have concluded that although for many elements of pathology both modalities have similar abilities, high-field scanners can depict lesions within the articular cartilage which would be difficult to identify using low-field imaging (Murray et al, 2009; Daniel et al, 2013). Comparatively, high-field MRI is associated with a shorter scanning time and a broader field of view, however, not without compromise, the sensitivity of the machine requires the patient to remain completely still, as even breathing can cause movement artefacts (Murray et al, 2009). Therefore, patients are required to be maintained under general anaesthesia, thus increasing the risk of injury and fatalities in the horse (Johnston et al, 1995; Senior, 2013).
Low-field MRI
The low-field MRI scanning system (<1 Tesla typically 0.27 Tesla) has been specifically designed to image the equine distal limb under standing sedation. ‘U-shaped’, the system allows the patient to stand astride the magnet, scanning each limb individually (Hallmarq, 2018; Mair et al, 2016) (Figure 3). This design enables scanning of structures as high as the carpus and tarsus, however movement artefacts are commonly seen in areas above the fetlock due to slight swaying of the patient (Mizobe et al, 2016). Nonetheless, it is the lower resolution ability of the low-field that allows for this movement while continuing to generate high quality images (Sherlock et al, 2009). Allowing general anaesthesia to be avoided is an obvious advantage, both from a patient safety aspect, but also a financial one. Typically, a low-field scan is considered to cost no more than a full lameness work up including nerve-block, ultrasound and radiography, minus the time and hazards associated with each. In comparison, the low-field system is more cost efficient to install and maintain with a higher number of installations within practices nationwide (Mizobe et al, 2016; Swagemakers et al, 2016).

Diagnostic application
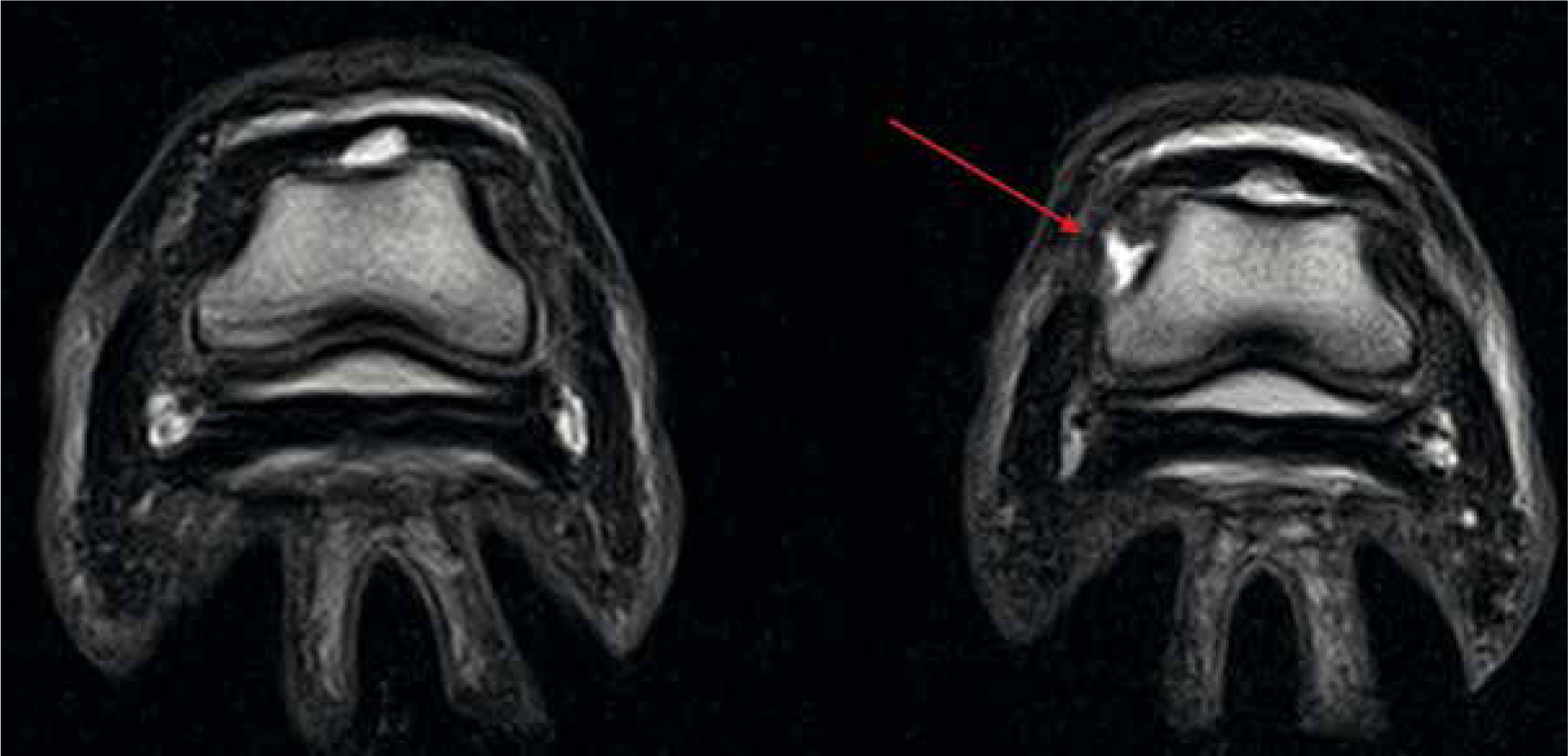
Given the anatomical composition and complexity of the equine limb it is no wonder that lameness is one of the most common problems seen in practice (Donnell et al, 2015). It is important that prior to proceeding with MRI the general location of the lameness source has been identified, this is often via nerve-blocks and radiographs in the first instance (Smith, 2015b). By doing so, the region of interest can be narrowed, reducing scanning time and thus sedation/general anaesthesia periods as well as mapping a concise scanning strategy (Daniel et al, 2013). As each patient is individual, it is common practice to image the limbs bilaterally in order to compare and identify what is anatomically normal for that patient. MRI is particularly well suited to distal limb pathology including orthopaedic and soft tissue injury (Figure 4). The proficiency to detect minute abnormalities in a structure include identifying single lobe damage within the suspensory ligament and pre-fracture pathology, which lends itself strongly to the care, training and maintenance of the competition horse (Daniel et al, 2013; Tranquille et al, 2017). This profound sensitivity allows prompt and accurate treatment to be prescribed and in turn promotes prevention or protection against further injury. Commonly, forelimb lameness is associated with damage to the fetlock and structures within the hoof, this ranges from subchondral remodelling to navicular oedema and everything in between (Biggi and Dyson, 2018). The level of detail required to identify the causes of lameness are often unobtainable by radiographs or ultrasonography alone, this can be multi-factorial, but often due to poor ultrasonic access to the capsule of the hoof and inadequate clarity or reduced radiographic contrast (Blunden et al, 2009; Mair et al, 2010).
Unit management
MRI systems need to be housed in a copper lined container or building in order to prevent outside radiofrequency interference, which can affect imaging quality and machine calibration. It is imperative the storage for the system is large enough to facilitate a protective perimeter around the magnet, known as the gauss line (Mair et al, 2010). This line represents the outermost boundary of which no ferromagnetic material from either the patient or technician should pass. Although largely known, patients or technicians with metal implants or cardiac pacemakers, for example, are unsuitable to enter the field due to the risk of electromagnetic interference and magnetic pull of the machine causing injury (Formica and Silvestri, 2004). Metal objects within the field should always be avoided, even when not in use, as this may damage the machine. All equipment associated with the use of the MR system must be compatible with the unit, this includes anaesthetic equipment, brushes, mops, etc, in order to prevent interference with calibration (Reddy et al, 2012). A strict hygiene protocol should be put in place between patients to not only minimalise cross contamination, but also maintain the equipment and check for damage. Although a high-field system uses liquid helium to conduct electricity and cool elements within the system, machines using 0.27 Tesla or less can be regulated via environmental temperature; therefore, ensuring the unit is maintained to within one degree via air conditioning and the room remains secure is imperative (Hallmarq, 2018).
Patient care
Patient compliance, behaviour and suspected region of pathology should be considered when choosing an imaging technique. Some patients, even once sedated, are not good candidates for standing MRI. An important factor in the management of the successful MRI patient is handling. Empathy and patience are vital attributes of the veterinary nurse in order to settle the patient into the situation and prevent stress; the calmer the horse the less resistance there is (Michou and Leece, 2012).
Patient preparation
General grooming should be undertaken prior to scanning to prevent any dirt, dust or bedding damaging the system. Whether under sedation or general anaesthetic, an intravenous catheter should be placed prior to the procedure for ease of venous access (Taylor et al, 2014). It is imperative all metal is removed from the hoof, this should include all nails and clenches, and is routinely checked via radiography as even a small amount of metal in a low-field system can cause significant signal interruption (Smith, 2015a). Depending on the site of investigation, it may only be necessary to remove the hind or forelimb shoes providing they do not pass the gauss line.
General anaesthesia
Prior to general anaesthesia horses are starved for 8–12 hours, however due to their physiology and predisposition to colic it is important to monitor them for signs of gut stasis and abdominal discomfort (Corley and Stephen, 2008; Nelson et al, 2013). In preparation for induction patients should have their mouths rinsed out with water and their tail bandaged, this prevents food debris being pushed into the trachea during intubation and the tail interfering with positioning and recovery. It is essential that all equipment to intubate, hoist the patient to the table, and connections to the anaesthetic machine are organised and that all personnel understand their role in this process (King, 2014). Once in position a urinary catheter can be placed to protect the machine, but also to void the bladder prior to recovery. The recovery phase, whereby the patient is often inaccessible, can take 30–60 minutes ideally. A wet floor, due to urination, will increase the risk of injury as well as soiling the patient and exacerbating the possibility of hypothermia (Clark-Price, 2013; Riley et al, 2016). Before commencing with the scan, the veterinary nurse must ensure the patient is adequately padded and supported, this will not only reduce movement artefacts from patient breathing (if done correctly), but will also reduce the occurrence of neuropathies and myopathies (Bidwell et al, 2007).
Standing sedation
Once accustomed to the new environment, the sedated patient may be walked or backed into the machine depending on the views required. It is vital, once positioned, the veterinary nurse ensures the horse is comfortable in order for them to be happy to stay still for the duration of the scan (Michou and Leece, 2012). Often a head rest and chest bar can be utilised to help the patient balance, relax, and prevent excessive swaying (Figure 5). Patients are required to evenly weight bear during the scan and, because they are lame, this can sometimes be difficult for them. Therefore, for patients that are deemed uncomfortable, the veterinary surgeon may administer an abaxial sesamoid nerve block to alleviate this issue, allowing the scan to commence without interruption (Carney et al, 2018). In a standing unit it takes approximately 1–2 hours (depending on scanning sequences, positioning, experience, and patient compliance) to complete each limb. Patients will often require a short break to stretch out and urinate once disturbed for repositioning the second limb. However, urine output is commonly increased due to the sedation or infused sedation method chosen. With this in mind, some patients may not manage to wait until the break, and ensuring the unit has a method of ‘emergency’ urine collection is key (Michou, and Leece, 2012). More commonly seen in geldings (but not limited to) occasionally the patient will not fully empty their bladder causing multiple issues: as well as disturbing the horse and causing them to reposition themselves, there is also a risk to the unit. In these instances, it may be appropriate to pass a urinary catheter (Morgan, 2016).

Post-procedure care
While the patient is still metabolising the pharmaceuticals used their temperature and mobility should be monitored, and nursing interventions completed such as bandaging the distal limbs and rugging the patient. By doing so, this protocol can reduce the instance of oedema in the limbs and hypothermia of the patient (Bennett, 2017; Riley et al, 2016). Both methods of scanning elicit sweating and long periods of immobility, so ensuring a return to normal temperature, movement and gut motility is imperative (Morgan, 2016). It is fundamental after general anaesthesia, to ensure the horse is reintroduced to food appropriately to prevent gastrointestinal impactions. During this period small amounts of soaked hard food, known commonly as ‘mash’, should be given repeatedly until the patient is fully conscious and ideally has passed faeces, the patient may then be provided their normal forage such as hay/haylage. Although uncommon, in the event of prolonged gut stasis, a nasogastric tube may be passed to facilitate administration of electrolytes and fluids to promote gastrointestinal motility and hydration (Jago et al, 2015; Bailey et al, 2016).
Conclusion
Given the safety, availability, and accurate diagnosis attainable via MRI it has become one of most valuable pieces of diagnostics equipment accessible in practice. Cost effective and with a multi-modal ability to identify soft tissue and osteopathology, MRI is sensitive enough to depict evidence of pre-fractural damage, hairline fractures and direct imaging of cartilage pathology minus the ionising radiation risk to both technicians and patient. Both high and low-field scanners have their individual advantages, the choice between them should consider region of interest, cost, patient status, behavioural attribute and owner understanding of the risks associated with both.
When managing the unit, it is imperative the temperature is controlled, hygiene is maintained, and the unit is protected from external radiofrequency interference. Patient support, positioning, restraint, and recovery are vital elements underpinning the nurse's role, ensuring the patient returns to normal gut mobility and temperature. With an increased amount of public information regarding veterinary technology available and depicted in the media, attitudes towards advanced medicines are altering. Paired with access to facilities, MRI has enabled clinical advancements in equine lameness and osteopathology to be made, providing accurate diagnosis and concise treatment.

