Fish, like any other animal, have disease representatives from all the major categories of aetiologies including viruses, bacteria, fungi, protozoa, metazoa, nutritional and environmental. By far the most common reasons for ill health in ornamental fish are poor water quality, ectoparasitism and bacterial infection. These conditions are relatively simple to diagnose and treat with all the equipment that are already available in veterinary clinics. There is sufficient information now available and there is a growing interest in the market to qualify consulting a veterinarian for fish health issues.
Collecting a history
House calls for fish cases are the preferred method for investigating fish diseases for several reasons including the ability to view the fish in its natural environment, having access to chemicals the owners had used in the past, observing the stocking density and aquaria setup.
If it is not possible to make a house call, then the following data set would be useful to obtain and the reader may notice some parallels with obtaining a history for other animals in the clinic:
Other pertinent questions include:
Water quality
Water quality is one of the most important factors influencing fish health. Often, clients attest that their water quality is fine but this is commonly due to nothing more than a visual assessment and possibly a pH test. The minimum database of water quality parameters to check includes water temperature, total ammonia nitrogen, nitrite, nitrate, pH, carbonate hardness (KH) or temporary hardness, which is a measure of the buffering capacity of the water against pH fluctuations, and general hardness (GH) or permanent hardness, which is the measure of divalent cations such as calcium and magnesium ions. In specific cases, testing for dissolved oxygen, dissolved carbon dioxide and chlorine levels may be necessary. In marine aquaria, testing salinity or conductivity, and calcium levels may be necessary. Sometimes it may also be necessary to examine water from the source and outflows. Many aquarium test kits are based on the addition of several chemicals to the test water to bring about colour changes which can be read against charts (Figure 1). Test strips and probes are also available.

Physical examination
A visual assessment of the fish from afar is the first step in examining a fish. This is so that the fish's behaviour is not altered by the presence of the observer. This part of the examination includes observing their behaviour and in parentheses are some of the differential diagnoses that would be associated with the pattern:

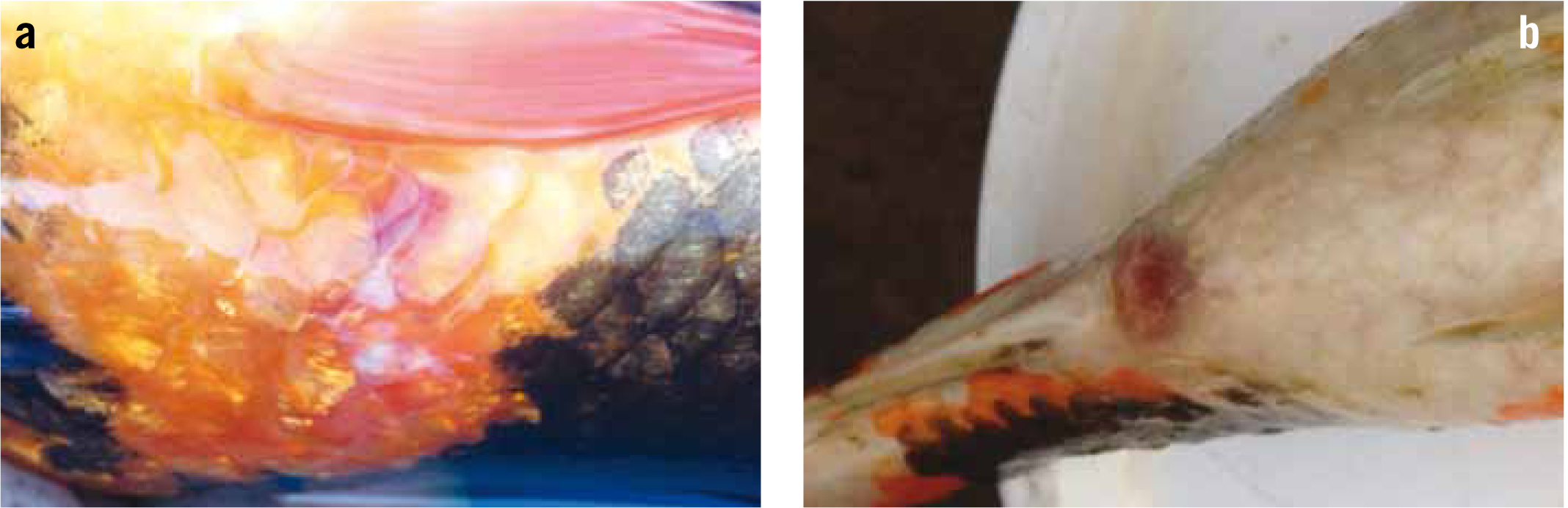
Once their behaviour has been fully assessed, then a physical examination can be performed. Here the examiner will check for signs of colour change, skin lesions (Figure 3a), ulcers (Figure 3b), tumours, excess mucus cover. Check for protruding eyes, cataracts, body condition, signs of ascites (bloat), subcutaneous oedema (otherwise known as dropsy (Figure 4a), gill colour and any areas of hyperaemia, paying particular attention to the eyes, vent (Figure 4b) and ventral surfaces. Check also for the presence of large metazoa such as fish lice and anchor worm.

At this point some non-lethal diagnostic procedures can be routinely undertaken such as a gill biopsy and skin mucus scrapes (see section on Microscopic examination). These procedures are usually performed on conscious restrained animals since an-aesthetics used for sedation may cause some ectoparasites to leave the host.
Microscopic examination
Two highly diagnostic samples to take from fish are a skin mucus scrape and a gill biopsy. These two samples will make it possible to diagnose all ectoparasitic conditions (protozoa (ciliates, flagellates), crustaceans, trematodes) and some external bacteria and fungi.
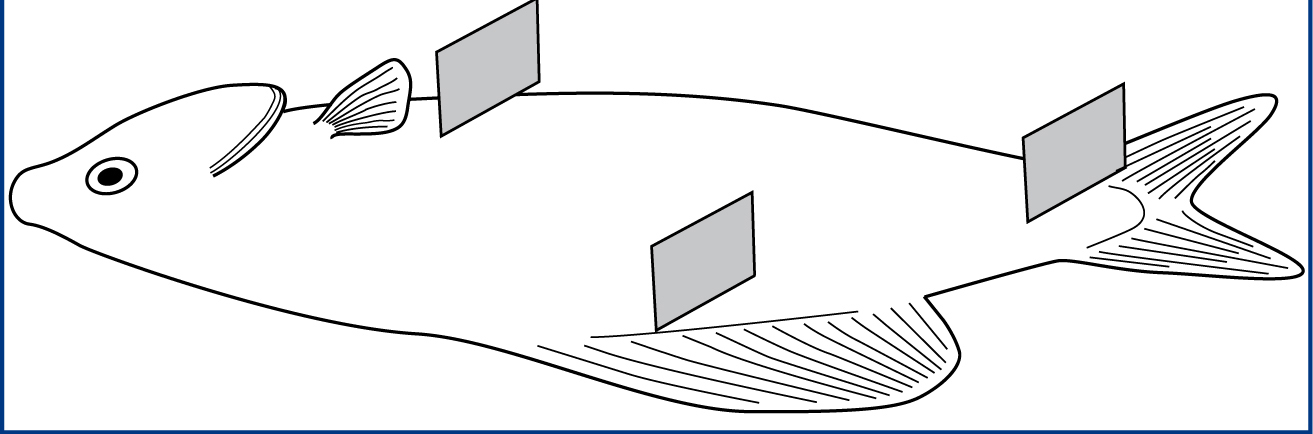
Mucus can be collected from three sites with gentle pressure, namely caudal to the pectoral fin, at the base of the dorsal fin and at the caudal peduncle (Figure 5). These are areas that the fish cannot scratch and dislodge the parasites. The author uses a glass cover slip for sample collection because it is easier to locate the mucus along the leading edge of the cover slip when viewing under the microscope. Some fish such as oscars produce copious amounts of mucus when caught, while some fish like the Tropheus have limited mucus cover and a scraping sound may be heard during sampling.
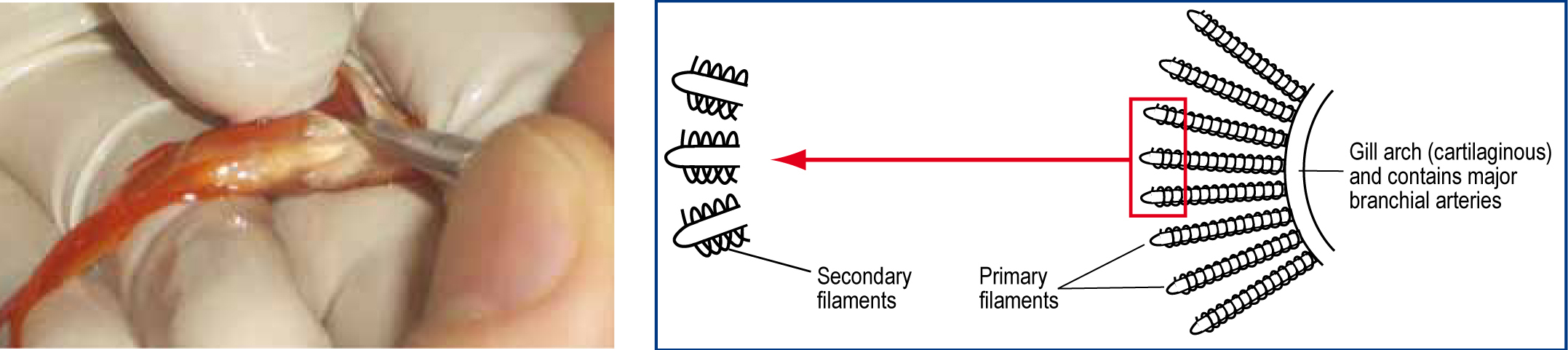
Gill clip of tips of several primary lamellae should be examined for parasites within the gill parenchyma and in the mucus. To do this, the fish should be held in a net, and iris scissors used to lever the opercular covering. The operculum should be held open by sliding the thumb gently towards the operculum, wedging it in place.
The tips of some primary filaments should be selected for biopsy. Only a small sample is required (3-5 filaments). To increase the chance of detecting parasites, small samples should be taken from multiple fish rather than large samples from fewer fish (Figure 6).
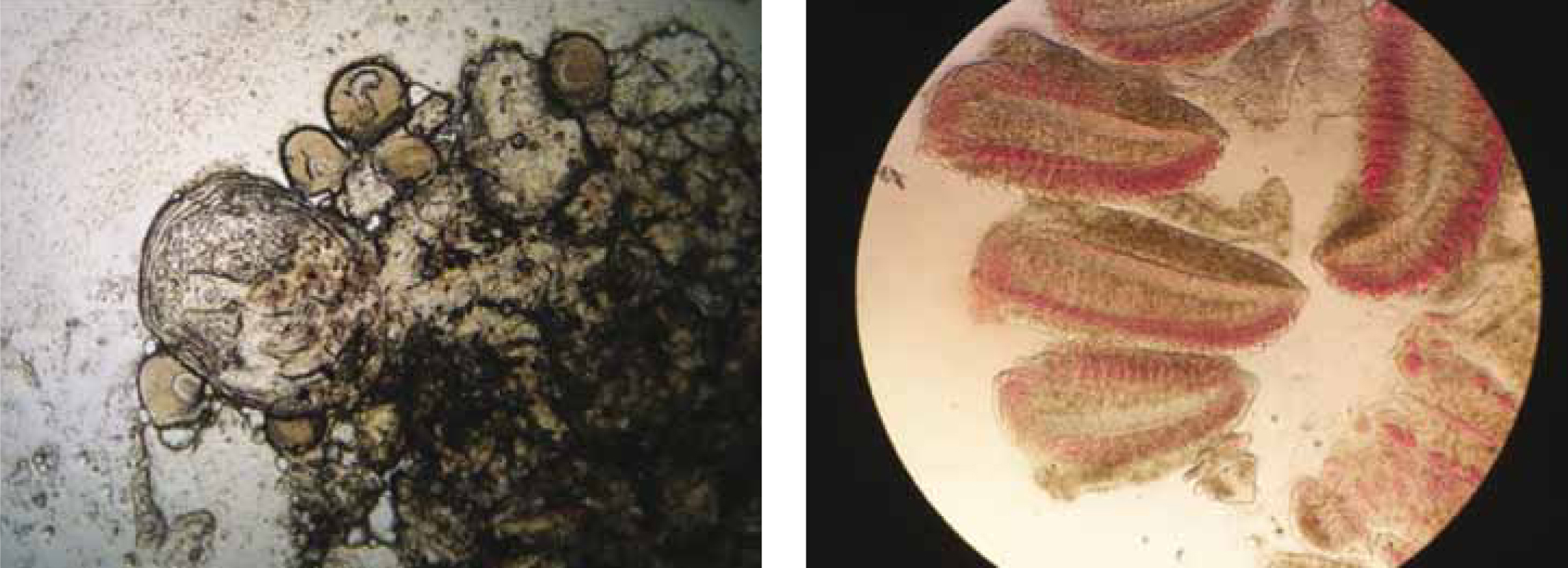
Wet preparations of freshly voided faeces are also useful to examine for intestinal flagellates and for ova of intestinal parasites. To encourage fish to void faeces, the client should be asked to feed the fish and shortly after, transfer it to a bare container so that any faeces voided will be easy to detect and pick up with a syringe or pipette.
Parasites are best observed live, via light microscopy, as they are easier to detect when motile. As such, wet preparations should be examined immediately as their style of locomotion is of high diagnostic value (Figure 7).
Sampling for laboratory testing
Bacteriology
When a bacterial aetiology is suspected, antibiotic therapy needs to begin immediately, however, it is advisable to take a bacteriology sample prior to medicating. This is in the event that bacterial culture and antibiotic sensitivity is required.
Samples for bacterial culture from skin wounds should be taken at the margin of an acute lesion. In individual fish with septicaemia, a sample of blood may be submitted for culture.
However, if large mortalities occur, it may be advisable to sacrifice a few fish for laboratory testing. To obtain material for culturing cases with bacteraemia, a swab of the kidney is the choice organ. It is a target organ in many diseases due in part to the extensive blood supply and it traps more than 70% of bacteria because of the large number of phagocytes lining the renal sinusoids. It is also the organ least likely to be iatrogenically contaminated when conducting the necropsy because it is situated in the retroperitoneal region, abutting the ventral aspect of the spinal column and covered by the swimbladder. In dead specimens, the swimbladder is removed carefully to reveal the kidney. In the laboratory, a pipette is inserted through the membrane and kidney material is aspirated. In the field, Amies swab with charcoal (MWE Medical Wire) is the ideal for obtaining a sample (Figure 8). Amies transport media is ideal for the recovery of aerobes and anaerobes and the charcoal enhances recovery of fastidious microorganisms.
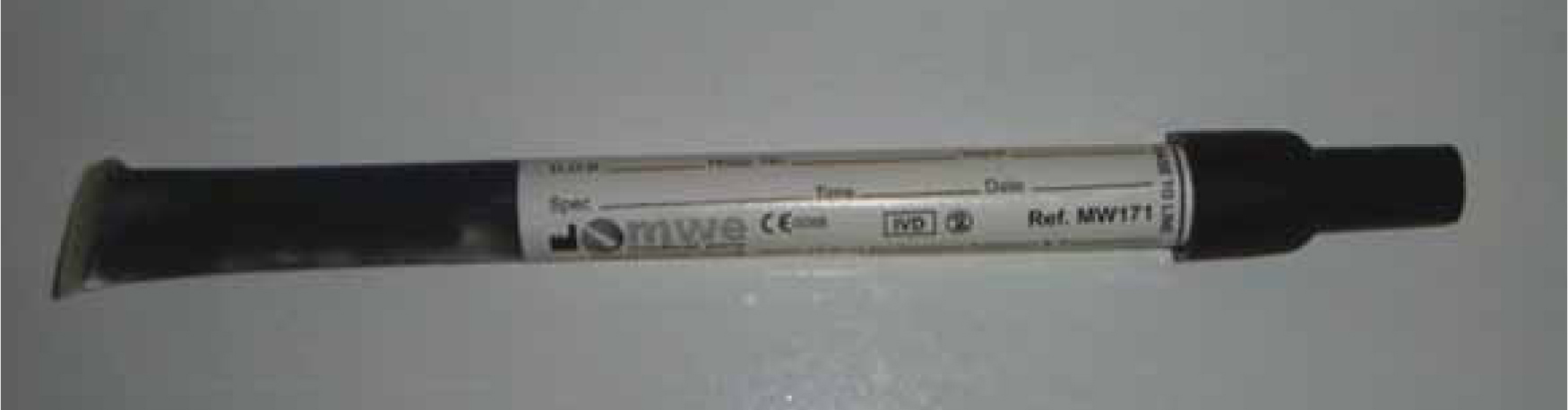
In very small, dead fish, the skin is decontaminated with antiseptic, dried with a sterile gauze swab and the back of the fish is cut, taking care not to enter the coelomic cavity. A sample can be taken from the area of haemorrhage (this should correspond to the location of the kidney since it is a well-vascularised organ).
A non-lethal method to obtain kidney material in live, anaesthetised fish is by using the percutaneous kidney biopsy technique. To access the kidney, the operculum is lifted, and a 3 ml syringe with a 22 G, 1 inch needle is inserted through the medial membrane of the gill chamber (just caudal to the last branchial arch) and directed dorsally, and then dorsocaudally into the kidney. A sample as little as 0.1 ml will be sufficient for culture. Confirmation that material has been successfully collected is by visualising melanomacrophages in a wet mount preparation. However, this technique is only appropriate in fish where there is kidney bulk anteriorly. The kidney of Cyprinids for example, may be better accessed surgically, via a paramedian approach.
Haematology and serum biochemistry
Haematology is important to identify blood parasites, bacteraemia and to qualitatively examine erythrocytes (e.g. cytoplasmic vacuolation seen in chlorine toxicity), and leucocyte counts and proportions (Figure 9). Neutrophils, monocytes, thrombocytes, eosinophils, basophils, mast cells and lymphocytes exist in teleosts and their functions resemble those of mammals. Leukocytes generally contribute to 10% of the total circulating blood cell population.
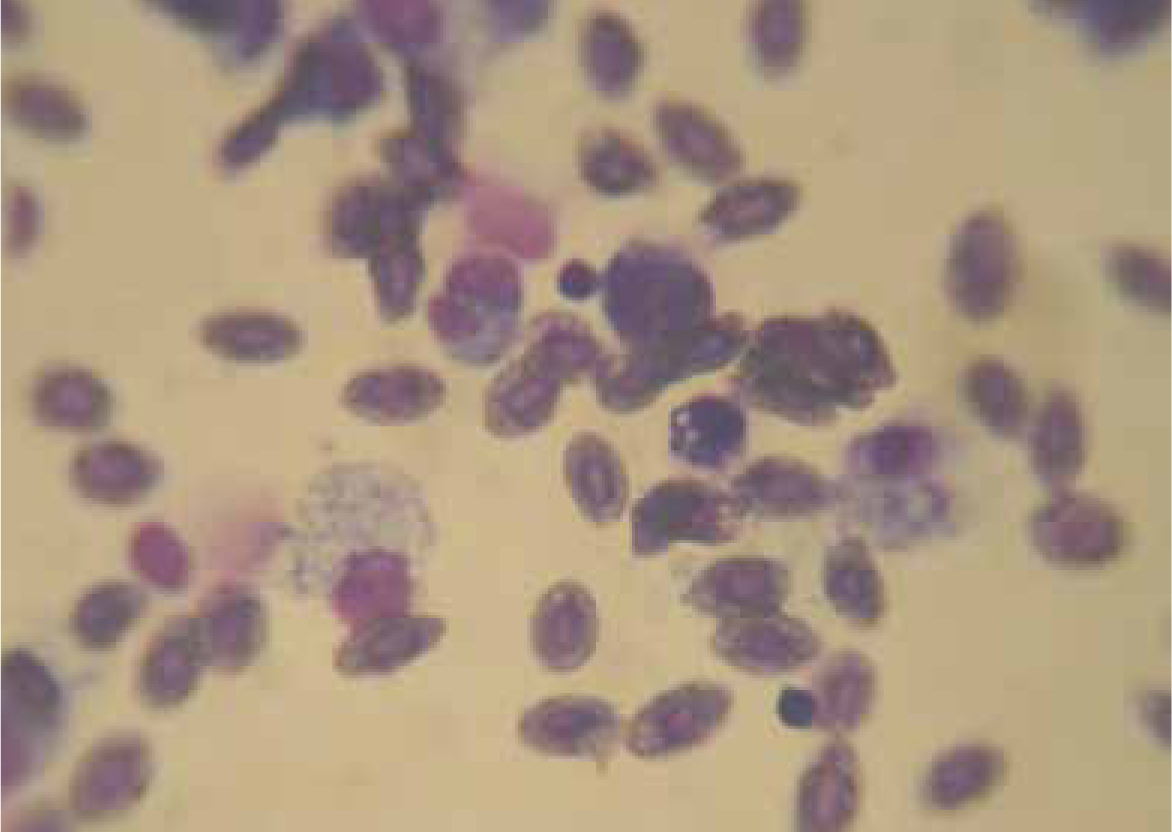
Fish with viral disease agent infections (e.g. salmon with infectious haematopoietic necrosis, infectious pancreatic necrosis, viral haemorrhagic septicaemia) show increase in total white cell count (WCC), lymphocytes and neutrophils; and decreased red blood cell count (RCC), haemoglobin (Hb) and haematocrit (Hct), mean corpuscular haemoglobin concentration (MCHC) and total protein. Ethylenediaminetetraacetic acid (EDTA) is the anticoagulant of choice when collecting blood for haematology.
For biochemistry testing, blood should be collected into heparin and chilled at 4°C. Samples must be centrifuged and processed within 1-2 hours of collection (since many enzymatic reactions in fish can still occur even at low temperatures). Reference values have not been established for many species because values may be influenced by the maturity, gender, season and other factors.
Fish should be heavily sedated for blood collection. The method for collecting blood in large fishes is similar to the technique used for cattle — i.e. via the tail vein (Figure 10). The fish should be held in dorsal recumbency and the needle is inserted at the ventral midline, at approximately 45° angle towards the anterodorsal direction, halfway between the vent and the caudal peduncle. The needle is inserted until it makes contact with the vertebral column where the large blood vessel is situated and the syringe can be drawn. In fish that are laterally compressed, or if the needle is not long enough, the tail vein can be accessed laterally, but this is more difficult. A landmark for this approach is that the lateral line runs parallel to the spine and so the vein would be located parallel to and just ventral to this.

Blood smears should be prepared immediately and air dried to avoid artefactual changes, especially osmotic macrocytosis.
Necropsy and sampling for histopathology
On many occasions it is necessary to collect samples for laboratory testing. Post mortem on typically affected, freshly euthanised fish (fish that have been dead for even a few hours are often of no diagnostic value due to autolysis) will allow sample preservation for histopathology (using 10% formalin buffered formalin) and microbiology (transport swabs or direct plate inoculation). Gloves should be worn to minimise zoonotic potential of some fish pathogens (e.g. atypical mycobacteria).
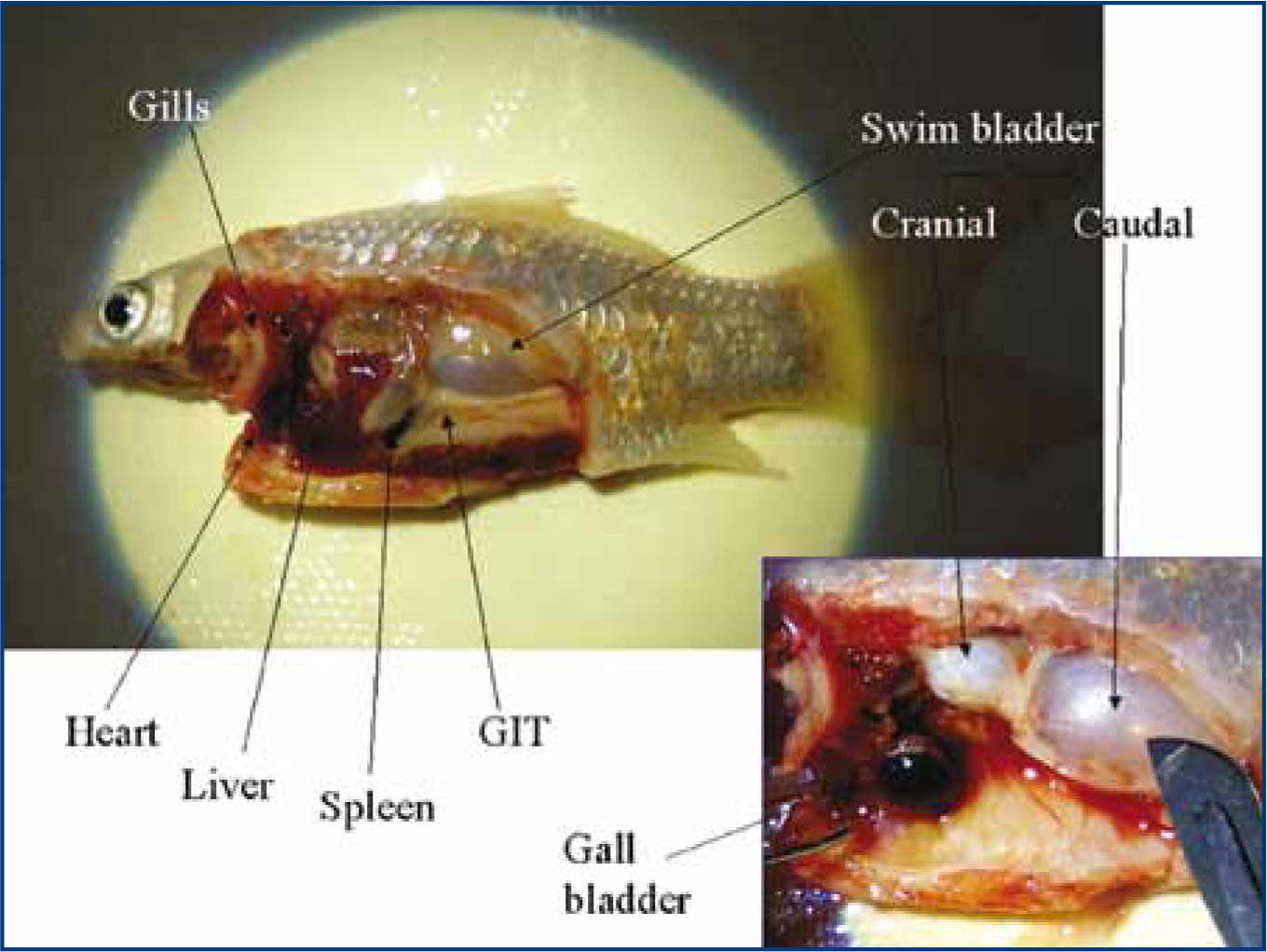
When conducting a necropsy, look for signs of haemorrhage, petechiation or ecchymoses, ascites, gut contents (whether it has recently eaten, or it may be inflamed), nodules/granulomas (especially in the kidney, spleen and liver), parasites, size of the gall bladder (large gall bladder means it has had a period of inappetance) and size of organs (enlarged spleens may indicate bacterial infection). Wet preparations of intestine can be done at this stage to visualise any intestinal flagellates.
In large fish, the tissues to collect for histopathology include: anterior kidney, posterior kidney, swim bladder, pancreas, liver, spleen, heart, gut, brain, gill (keep the primary filaments attached to the gill arch), skin (include any skin lesions with margin of healthy tissue; take care to avoid scale detachment). If in doubt about the organ type, then the author recommends taking one of everything (Figure 11). For small fish that are about 2 cm in length, it is sufficient to simply remove one opercular flap and one side of the abdominal wall before fixation. It is important to use sufficient volume of formalin (1:10 tissue to formalin) to adequately preserve specimens for the laboratory (Loh and Landos, 2011).
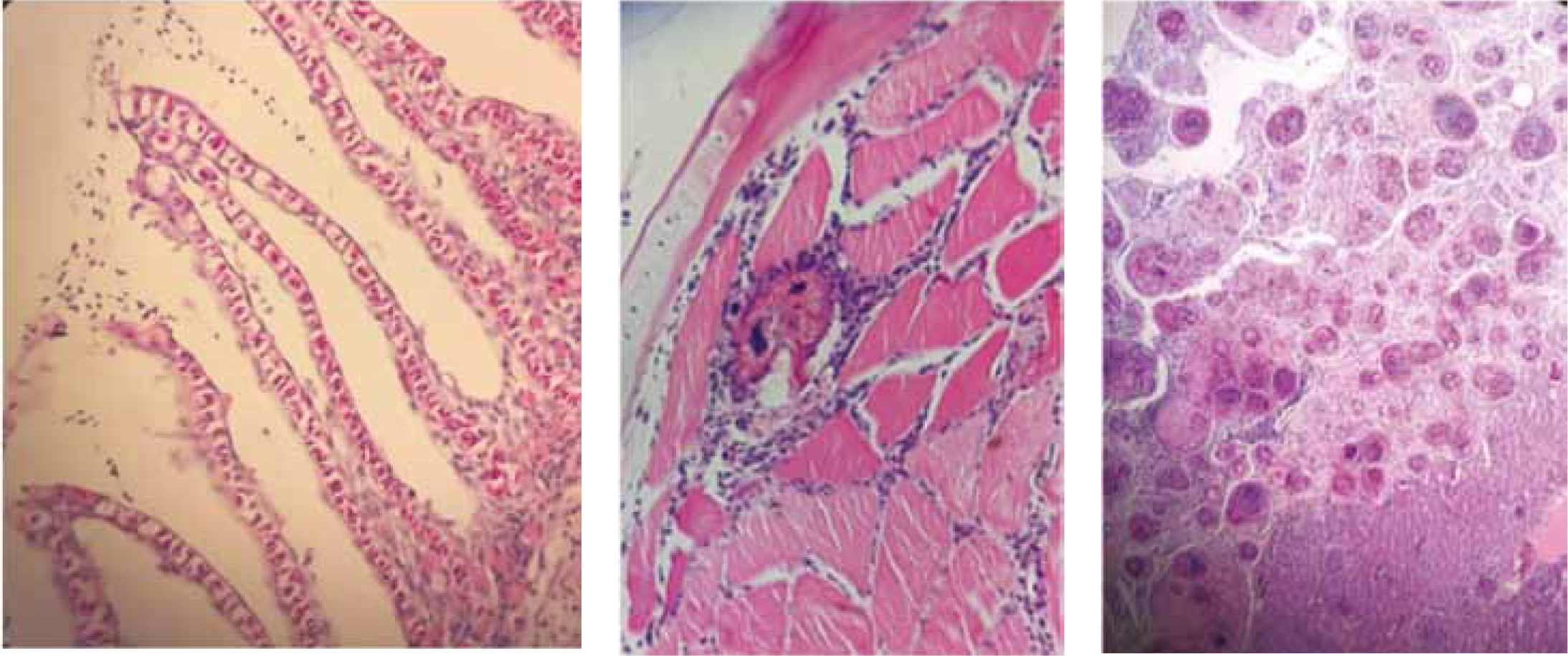
Histology is a powerful technique for investigating disease because it shows evidence of the disease process and will provide clues as to the aetiological agent (Figure 12).
Conclusions
The most common reasons for ill health in fish are poor water quality and ectoparasitism. With some perseverance, aquatics can be added to the list of species currently treated.
