Cardiopulmonary arrest (CPA) is the cessation of spontaneous ventilation and systemic perfusion leading to inadequate tissue oxygen delivery and death. Cardiopulmonary cerebral resuscitation (CPCR) is a technique utilized to reverse CPA. CPCR can be divided into three stages: basic life support; advanced life support and post-resuscitation care. This article provides a review of how to provide effective advanced life support after basic life support has been initiated and highlights the importance of post-resuscitation care in order to optimize the chance of the patient being discharged from hospital with a good quality of life. Recognition of CPA and basic life support has been discussed in a previous article (Archer, 2011).
Advanced life support
Advanced life support concentrates on identification and treatment of arrhythmias, and drug and fluid therapy to support circulation. The steps can be remembered by the ‘DEF’ mnemonic (D, drugs; E, electrical activity of the heart; F, fluid therapy) following on from the ‘ABC’ of basic life support. While it is the job of the veterinary surgeon to select appropriate drugs, nurses should have an understanding of the drugs that may be required and the effects they exert.
Drugs
Drugs administered during CPCR include: those used to improve heart rate, increase myocardial contractility, vasoactive drugs which improve blood pressure by vasoconstriction, or antiarrhythmic drugs. Drugs may also be used to antagonize effects of sedative drugs or opioids. Table 1 demonstrates drugs currently used during CPCR, however as stated by the human ECC committee, subcommittees and task force of The American Heart Association (2005), no medication has been shown to improve neurologically intact survival after cardiac arrest.
Table 1. Drugs administered during cardiopulmonary resuscitation (In alphabetical order)
| Drug | Effect | Indication during CPCR | Disadvantage |
|---|---|---|---|
| Adrenaline | Mixed adrenergic receptor agonist Peripheral arteriolar vasoconstriction leading to shunting of blood to brain, heart and lungs. Increased myocardial contractility and increased heart rate | Asystole, ventricular fibrilation (VF) | Increased myocardial oxygen demand. Vasopressor effect reduced by hypoxia and acidosis |
| Amiodarone | Class III antiarrhythmic. | Refractory VF after defibrillation and adrenaline, ventricular tachycardia, supraventricular tachycardia | |
| Atipamezole | Antagonizes the effects of alpha-2 adrenergic receptor agonist drugs | If alpha -2 agonist drug administration is suspected to be the cause of CPA or if alpha 2 agonists had been recently administered prior to arrest | |
| Atropine | Parasympatholytic, increases heart rate | Bradycardia, vagally induced asystole | Increased myocardial oxygen demand, tachycardia |
| Flumazenil | Antagonizes the effects of benzodiazepines | If benzodiazepene drug administration is suspected to be the cause of CPA or if it had been recently administered prior to arrest | Expensive |
| Lidocaine | 1b antiarrhythmic | Ventricular tachycardia, supraventricular tachycardia | Decreased ROSC and asystole after defibrillation compared with amiodarone. Makes electrical defibrillation more difficult |
| Naloxone | Antagonizes the effects of pure opioid agonists | If full opioid drug administration is suspected to be the cause of CPA or if opioids had been recently administered prior to arrest | Also antagonizes analgesic effects |
| Sodium bicarbonate | Buffer | Severe pre-existing metabolic acidosis, hyperkalaemia and tricyclic antidepressant overdose | Not for routine CPA use. Best treatment for acidosis due to CPA is to maximize ventilation and perfusion |
| Vasopressin | Specific non-adrenergic receptor (V1A) agonist in smooth muscle of the vasculature resulting in vasoconstriction | Asystole, ventricular fibrillation. Preferential shunting of blood to central nervous system and heart. May improve cerebral perfusion | Expensive, may be difficult to get hold of |
CPCR, capulmonary cerebral resuscitation; ROSC, return of spontaneous circulation; CPA, cardiopulmonary arrest
Routes of administration
As suggested by Kruse-Elliot (2001) and Plunkett and McMichael (2008), the intracardiac route of administration should be avoided due to the risk of coronary vessel laceration, cardiac tamponade, pnuemothorax and myocardial ischaemia. Plunkett and McMichael (2008) recommend intravenous (IV) administration of drugs or the intratracheal (IT) route if an intravenous catheter is not already in place. They suggest that a central IV catheter is preferred, however, as one is rarely placed prior to CPA, a peripheral catheter is commonly used. Peripheral injections of drugs should always be followed by a 0.9% saline flush and they recommend raising the limb for 10–20 seconds after injection to encourage the drug to reach the central circulation. It takes 1–2 minutes for drugs administered peripherally to reach the central circulation and the human ECC committee, subcommittees and task force of The American Heart Association (2005) recommend continuing chest compressions for 2 minutes after drug administration before electrocardiogram (ECG) evaluation. Plunkett and McMichael (2008) suggest that atropine, adrenaline, lidocaine, naloxone and vasopressin can be administered via the IT route, however the dose of drug needs to be increased by 2–2.5 times (adrenaline up to 10 times) compared with IV injection so dose charts should be prepared accordingly. They recommend diluting the medications with 2–5 ml of sterile water to improve distribution of the drug. Cole et al (2002) suggest the drugs should be administered via a dog urinary catheter down the endotracheal tube advanced tothe carina. Plunkett and McMichael (2008) suggest intraosseous drug administration may be useful in cases where IV access is unavailable, especially if fluid therapy is required. Suitable sites include the tibial crest, the proximal humerus and the femoral greater trochanter.
In the author’s experience, keeping a drug dosage chart with commonly used emergency drugs allows rapid preparation of drugs required in an emergency. Syringes should be clearly labelled once drawn up to prevent confusion and time wasting although should be discarded if unused. Pre-loaded syringes can also be purchased which can be kept for longer.
Electrical activity of the heart
It is the veterinary surgeon’s job to diagnose and treat arrhythmias, however nurses should be prepared to place an ECG and be able to alert the vet to abnormal rhythms. Abnormal rhythms detected on ECG causing pulseless cardiac arrest include: pulseless electrical activity (PEA) (Figure 1), asystole, ventricular fibrillation, and pulseless ventricular tachycardia. In a study by Rush and Wingfield (1992), PEA was shown to be the most common ECG rhythm seen during CPA (seen in 23.3% of all canine and feline arrests). Asystole was the second most common, seen in 22.8% of cases and third most common was ventricular fibrilation, seen in 19% of cats and dogs in CPA (Figure 2). Bradycardia was observed in 19% of dogs and cats during CPA.
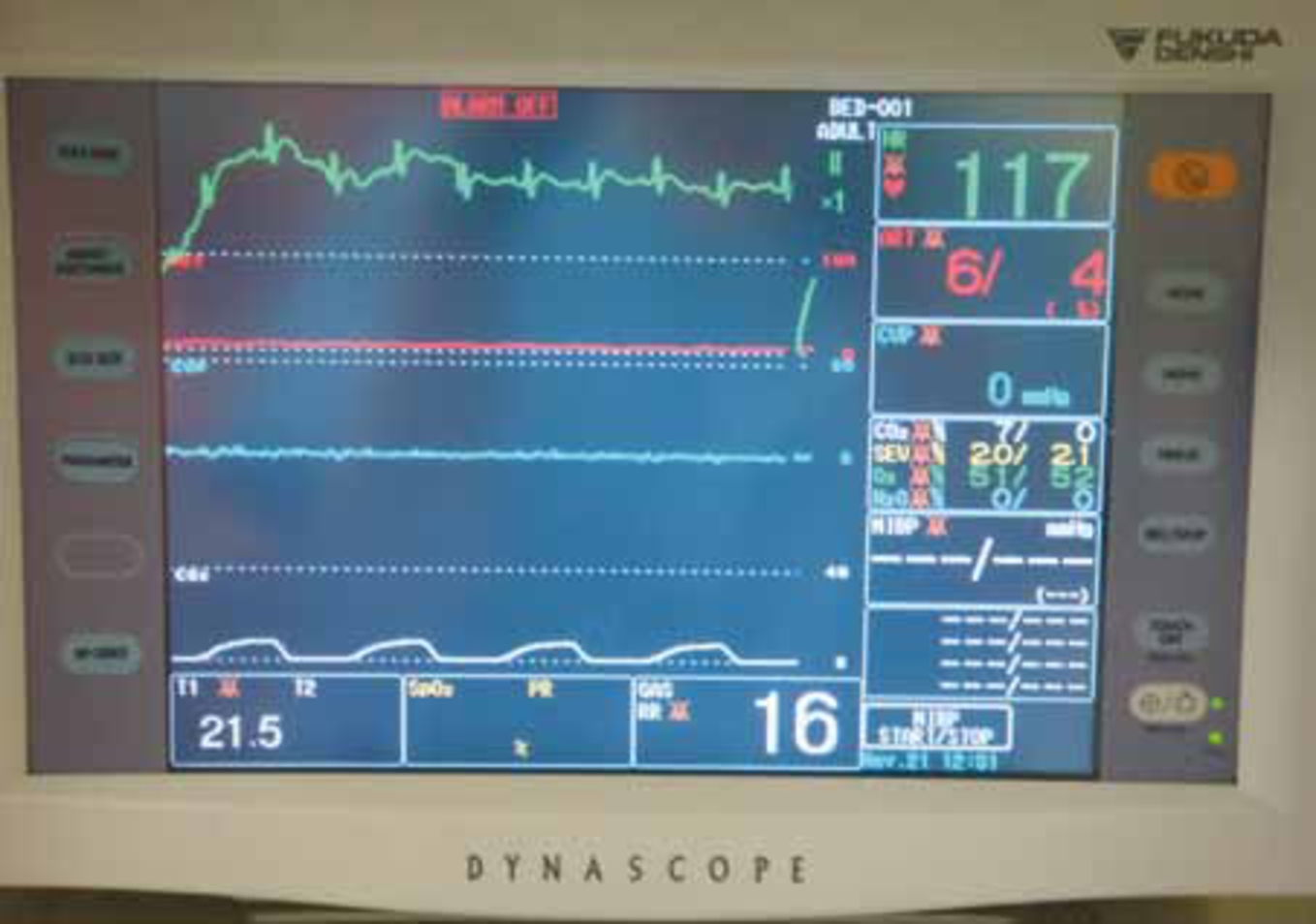
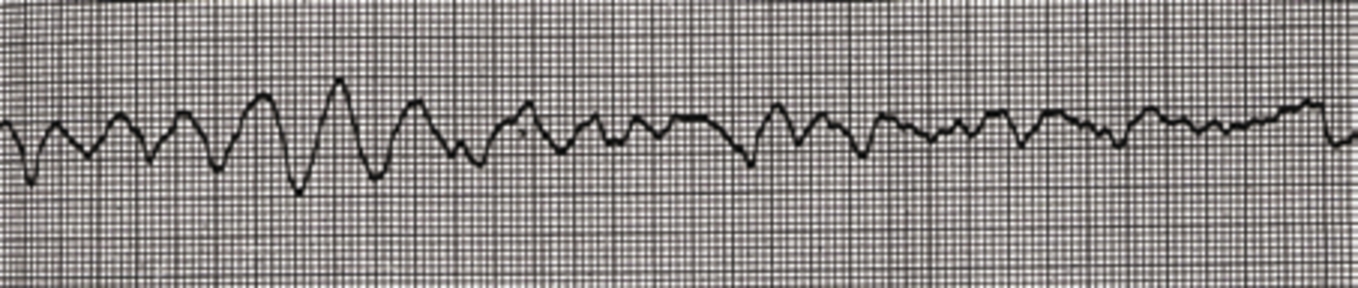
Defibrillation
Electrical defibrillation is required for patients in ventricular fibrillation (Figure 3). A dose of 3–5 joules/kg is widely recommended initially and sufficient conductive gel should be used. Plunkett and McMichael (2008) recommend that chest compressions are continued for another 2 minutes after defibrillation before re-evaluating the ECG for signs of normal sinus rhythm. As stated by Cole et al (2002), the energy should be doubled if a second defibrillation is required. If a debrillator is available in practice, nurses should ensure all staff are trained in its correct use and in defibrillator safety.

Fluid therapy
Kruse-Elliot (2001) advocates routine use of shock doses of crystalloid intravenous fluid therapy in veterinary CPCR, however both recent veterinary CPCR review articles (Cole et al, 2002 and Plunkett and McMichael, 2008) recommend that these high doses recommended by Kruse-Elliot (90 ml/kg for dogs and 45 ml/kg for cats) should be reserved for patients with pre-existing hypovolaemia or significant ongoing losses. Their recommendations are based on a canine study which showed that excessive crystalloid therapy decreased cerebral and myocardial perfusion (Ditchey and Lindenfeld, 1984). This is supported by Van Pelt and Wingfield (1992) who also suggest that fluid administration during CPCR should be conservative unless hypovolaemia is the reason for CPA. Instead, for the normovolaemic CPA patient administration of a 20 ml/kg crystalloid bolus for dogs (10 ml/kg bolus for cats) is recommended as rapidly as possible (Plunkett and McMichael, 2008).
Post-resuscitation care
Management of CPA does not end at return of spontaneous circulation (ROSC). Close monitoring of the patient’s cardiovascular, respiratory and neurological systems are important for at least the first 24 hours post arrest. Patients require intensive nursing, supportive care and monitoring to optimize blood pressure, oxygenation and ventilation so that vital organ perfusion in maintained and the risk of complications minimized. The underlying cause for arrest should be found, with specific treatment instigated to address any abnormalities. Cardiopulmonary or respiratory re-arrest is common after successful CPCR: a study by Wingfield and Van Pelt (1992) demonstrated that 69% of dogs which were intially resuscitated successfully experienced a second CPA. Common complications seen after CPCR include cerebral oedema, hypoxemia, gut reperfusion injury, abnormal hemostasis, acute renal failure, sepsis and multiple organ dysfunction syndrome due to reduced perfusion during CPA (Plunkett and McMichael, 2008). Nurses should communicate with the veterinary surgeon about whether resuscitation is to be attempted if rearrest occurs.
Basic nursing care
Very little literature is available with regards to nursing care specifically in the post-resuscitation period, however many basic nursing principles can be applied. Initially the patient is likely to be depressed and have altered mentation, or may even be unconscious. If the patient has a reduced palpebral reflex then ocular lubricant should be applied regularly to prevent ocular ulceration. The risk of oral ulceration should be minimized by regular moistening of the tongue and the mucous membranes with water. The patient should be turned frequently (every 4 hours in the author’s experience) to prevent decubital ulcers and to minimize the risk of atelectasis (Leece and Hill, 2003). Propping the patient into sternal recumbency allows uniform lung expansion. Adequate padded bedding should be provided. Massage and gentle physiotherapy, such as ‘range of movement’ exercises, should be performed on recumbent patients. Good, detailed record keeping is vital and makes shift changeovers much easier.
Monitoring
Fluid balance
Fluid balance should be monitored in the post-resuscitation period. The measuring of fluids going into the patient and fluids coming out of the patient (i.e. urination), often referred to as ‘ins and outs’, is valuable to guide fluid therapy. The total ins and outs can easily be recorded on the hospital sheet at the end of each day to check they balance. An indwelling urinary catheter is useful in the post-resuscitation period as it allows urine output (UOP) to be monitored to assess adequacy of fluid intake. It also helps keep the patient clean and dry and prevents urine scalding. UOP and urine specific gravity (USG) gives an indication of the adequacy of fluid therapy, the hydration status and renal function of the patient. Normal UOP is 1–2 ml/kg/hour ((Leece and Hill, 2003). Anuria is defined as a UOP <0.5 ml/kg/hour and should be addressed immediately with increased intravenous fluid therapy. If the patient does not have a urinary catheter then urination can be estimated by weighing bedding or catching urine in a dish when expressing the bladder.
Hydration and volume status can also be assessed using the peripheral pulse rate and quality, mucous membrane moistness, the core-peripheral temperature gradient and other laboratory tests discussed later.
Cardiovascular system
Continuous cardiovascular monitoring is necessary during the post-resuscitation period. Peripheral pulses should be palpated to assess pulse quality, rate and rhythm. Peripheral rather than central pulses should be used as these give a better indication of peripheral blood flow (Leece and Hill, 2003). The mucous membranes should be examined and should be pink and moist, with a capillary refill time of <2 seconds. Cardiac auscultation is used to assess the heart rate and rhythm and demonstrates the presence of murmurs. The pulse should be palpated simultaneously. The ECG is useful to identify arrhythmias, which may be seen in the post-resuscitation period due to direct myocardial trauma from chest compressions, hypoxia, electrolyte disturbances, pain, drug administration, or re-perfusion injury, and to monitor efficacy of arrhythmia treatment. Blood pressure measurement is a useful guide to cardiac output in the post-resuscitation period. In the author’s experience a Doppler blood pressure monitor may be preferable to the oscillometric machines which may not work in the post-resuscitation period if there is poor peripheral perfusion. The Doppler is also useful as it produces an audible pulse, however if peripheral perfusion is very poor or the patient is extremely vasoconstricted then the Doppler will not be able to detect the pulse either.
Respiration
Oxygenation and adequacy of ventilation can be judged by assessing respiratory rate, depth and pattern and mucous membrane colour. If available, arterial blood gas measurement can be used to measure the arterial partial pressure of oxygen (PaO2) and carbon dioxide (PaCO2). A pulse oximeter can be used to measure the percentage of haemoglobin saturated with oxygen (SpO2), which should be maintained >90% to avoid hypoxaemia. If hypoxaemia occurs, oxygen should be supplemented (Egger, 2007). Oxygen can be supplemented using face-masks or ‘flow by’ temporarily, but nasal prongs or a nasal catheter may be required for longer-term supplementation. Patients unable to ventilate adequately should have their trachea intubated and lungs ventilated. The adequacy of ventilation should be guided by the end tidal carbon dioxide (EtCO2) with the aim to maintain EtCO2 in the normal range of 35–45 mmHg. Both over and under ventilation should be avoided as this can affect cerebral perfusion (Leece, 2007).
Laboratory tests
Lactate is produced by tissues undergoing anaerobic metabolism. Poor tissue perfusion during and after CPCR leads to lactic acid build up and metabolic acidosis. If a blood gas analyzer is available, the acid–base status of the patient should be checked. The sample should be taken anaerobically and analyzed as soon as possible. Serum lactate can also be measured to assess adequacy of tissue perfusion; increased lactate indicates poor tissue perfusion. Normal blood lactate concentrations are <2.0 mmol/l in dogs and <1.6 mmol/l in cats (Mathews, 2006). Packed cell volume and total protein are useful in the post-resuscitation period, particularly to assess hydration status and if haemorrhage is suspected. Serum urea and creatinine levels used in combination with UOP and USG indicate hydration status and kidney function. Electrolyte disturbances are common in the post-resuscitation period so should be monitored regularly where possible.
Neurological monitoring
Neurological dysfunction occurs commonly after CPCR; Plunkett and McMichael (2008) suggest this often resolves after 24–48 hours. Any situation that causes a rise in intracranial pressure such as vomiting, sneezing or head shaking should be avoided. Cerebral perfusion and oxygenation can be maximized by maintaining blood pressure and providing supplemental oxygen if required. The patient’s level of mentation, pupil size and pupillary light reflexes should be assessed regularly and the veterinary surgeon informed immediately if anything changes. Signs of increased intracranial pressure include: anisocoria, altered mentation, miosis or mydriasis and depression.
Body temperature
Body temperature should be monitored regularly and care taken with agressive warming devices. As stated by the ECC commitee, subcommitte and taskforce of the American heart associations (2005), hyperthermia should be avoided as it is associated with increased cerebral oxygen demand. In human CPA, mild therapeutic hypothermia has been shown to reduce mortality and increase the rate of a favourable neurological outcome (Bernard et al, 2002; Hypothermia after cardiac arrest study group, 2002). Plunkett and McMichael (2008) advocate ‘permissive hypothermia’ in dogs and cats. Permissive hypothermia is the term used when hypothermia is allowed to continue without taking steps to rewarm the patient to a normal body temperature without recommending active cooling. This is not without risks, as complications of hypothermia include arrhythmias, coagulopathy, respiratory depression and shivering which increases oxygen demand (Murison, 2001). In the author’s opinion further veterinary clinical studies are required in regards to the optimum body temperature and duration of hypothermia before its use is routinely recommended in the post-arrest patient.
Outcome of advanced life support
The decision to terminate advanced life support and to euthanaze the patient will depend on many factors such as the underlying disease processes, the owner’s wishes and the reponse to initial resuscitation attempts. Plunkett and McMichael (2008) recommend allowing a minimum of 48 hours before a prognosis is made regarding neurological outcome and suggest that signs correlating with a poor neurolgical outcome include absent corneal and pupilary light reflexes and absent motor response and withdrawl reflex to pain.
Recommendations for veterinary practice
The DEF of advanced life support should be executed efficiently in the event of CPA. Twenty four hour veterinary care and intensive monitoring is required to provide adequate post-resuscitation care and if these cannot be provided at a practice then transferring the patient to a nearby emergency clinic should be considered. Currently, the Reassessment Campaign on Veterinary Resuscitation (RECOVER) is in the process of generating the first evidence-based guidelines for CPCR in dogs and cats. Once published, nurses can use the information provided to train staff in current, evidence-based CPCR and practices can use these guidelines to draw up their own algorithms to follow in the event of CPA.
Conclusions
Advanced life support is a necessary continuation of basic life support and can easily be remembered by the DEF mnemonic. Staff should be trained properly in advanced life support as well as basic life support. Post-resuscitation care is an area where nurses can use all their skills to the maximum, however nursing these patients is hard work and time consuming, without the guarantee of a positive outcome. Basic nursing care for the recumbent patient must not be overlooked and as many parameters as possible should be monitored in order to build up the best possible picture of the patient’s condition.
Key Points
- Cardiopulmonary cerebral resuscitation (CPCR) does not end once spontaneous circulation has returned: the goal of advanced life support is to support the circulation and to maintain adequate organ perfusion in order to ensure survival to discharge from hospital with a good quality of life.
- The steps of advanced life support can be remembered using the mnemonic DEF (drugs, electrical activity and fluid therapy).
- Adequate post-resuscitation care and monitoring is an area where nurses can use all their skills to their full potential and basic nursing care must not be overlooked.
