In recent years, the threat posed to both pets and people by parasites has grown, fuelled by a milder climate and increased movements of pets and people. Nurses play a vital role in discussing parasite control and clients will sometimes feel more comfortable seeking advice in nurse clinics or at reception rather than in veterinary consultations where time may be limited.
In the UK, cat fleas and Toxocara spp. roundworms are present at high prevalence across the country, exposure to infection cannot be avoided and the clinical and zoonotic significance of infection is high. In addition, tick numbers and activity is increasing, Angiostrongylus continues to spread across the UK and hydatid disease may present a renewed threat though abattoir spread. This in combination with exotic parasites entering the country on travelled and imported pets has created a very fluid situation, with the distribution of parasites and risk of exposure rapidly changing.
Fleas
Cat fleas are thriving in the UK with recent mild winters and wet, warm summers allowing prolonged survival and favourable breeding conditions in outdoor environments. Central heating also allows environmental stages of the flea life cycle to persist all year round in the home. This combination of factors leads to increased flea challenge on domestic pets and without routine preventative treatment, there is a high risk of flea infestations establishing (Coles and Dryden, 2014). Although cat fleas cannot live and reproduce on people, they can bite leading to human irritation. They are also a source of revulsion, eroding the human–animal bond. They are a cause of flea allergic dermatitis, anaemia in heavy infestations and vectors for a variety of infections including Bartonella spp. (cause of cat scratch disease), Rickettsia felis (cause of spotted fever) and Haemoplasma spp. (cause of feline infectious anaemia).
Flea control is therefore vital to reduce disease risk and maintain a strong healthy relationship between pet and owner.
Diagnosis
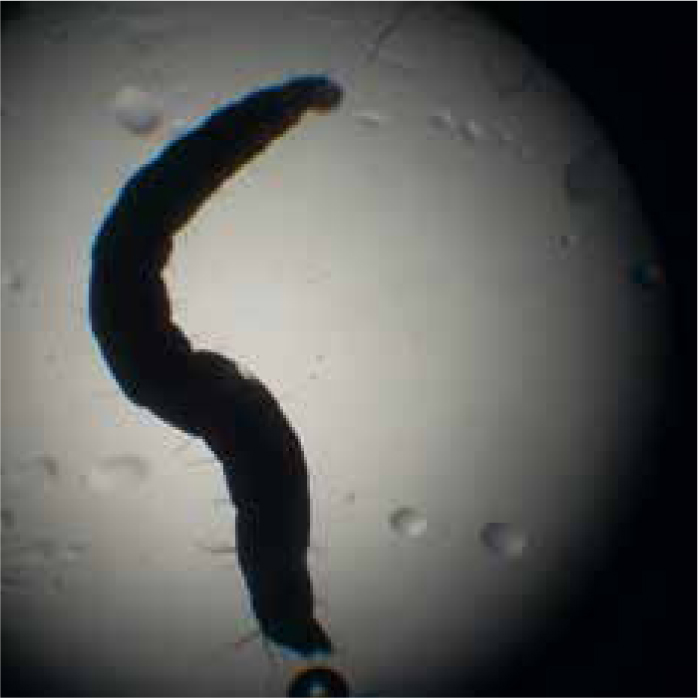
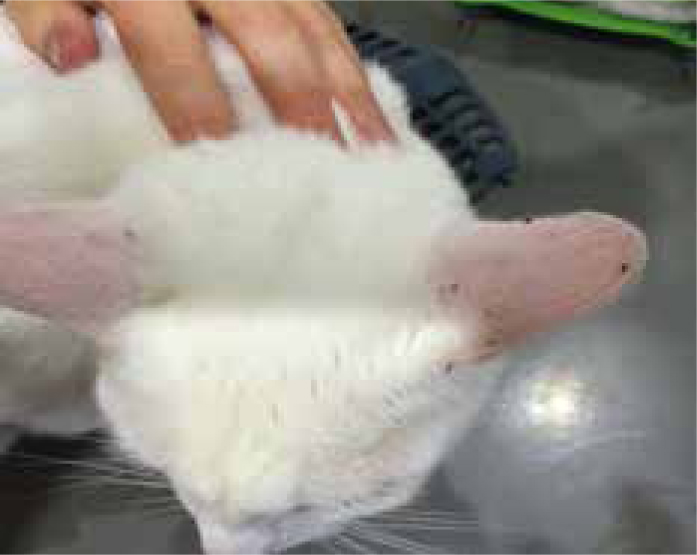
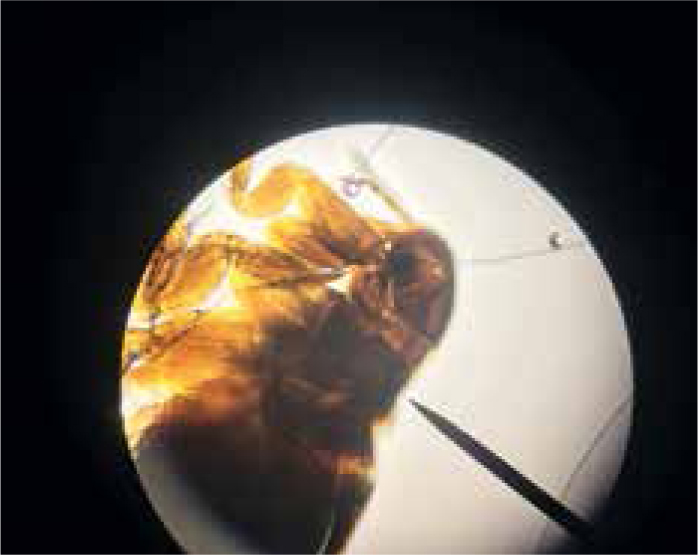
Diagnosis of flea infestations can be easily achieved, not only in clinical consults but also in nurse clinics. A flea comb can be used to find adult fleas, eggs or flea dirt (Figure 1). Flea dirt comprises mostly of partially digested blood and will smear red on damp cotton wool or can be identified microscopically. Clients will also sometimes find flea larvae (Figure 2) and present them for identification. Adult fleas found may be mounted in a drop of water under a cover slip and examined microscopically. It is important to identify what type of flea is causing an infestation if control is being lost. Cat fleas have both genal and pronotal combs and a characteristic elongated head, with the head being twice as long as it is tall (Figure 3). Flea control advice for household pets is centered around Ctenocephalides felis and treatment of all susceptible pets with an effective adulticide at the correct frequency, combined with treatment of the environment should be sufficient for control. Pulex irritans, the ‘human flea’ can be easily distinguished from cat and dog fleas as it has no combs and a rounded head, giving it a ‘bald’ appearance. Its presence in a household means that medical advice must be sought by pet owners as treatment of the owner as well as pets in the household will be required. Spilopsyllus cuniculi, the ‘rabbit flea’, possesses both genal and pronotal combs but the genal comb is short (only 4–6 spines) and oblique rather than horizontal. Adults are also smaller than other fleas infesting household pets with adult females typically only reaching 1 mm long and adult males being smaller. They characteristically congregate around the ear pinna and fleas found predominantly in this location should raise suspicion of S. cuniculi infestation (Figure 4). They are more sedentary than other species of flea found on domestic pets and household rabbits are uncommonly infested. Reproduction in the flea is controlled by rabbit hormones to ensure that flea mating and egg production occurs in the presence of young rabbits. It therefore, rarely infests homes, but cats and dogs can become incidentally exposed when hunting rabbits or when investigating warren entrances. Ceratophyllus gallinae, the ‘European chicken flea’ (Figure 5) has a pronotal but no genal comb. They are fleas of birds, with adult fleas living in nests and jumping on birds using the nest to feed. They overwinter as pupae and then feed again on the birds using the nest the following year. Houses may be invaded if nests are adjoining buildings and owners and pets subsequently bitten.

Nurses play a vital role in early detection of flea infestations in nurse clinics, not only for dedicated flea checks but also at kitten checks, behavioural and obesity clinics. Signs of irritation, alopecia, dermatitis and visible flea dirt can be picked up on, demonstrated to the client, and control measures implemented.
Flea control measures should be recommended to all clients as even indoor cats may be exposed through mechanical transmission of fleas on clothes or through stray cats or wildlife bringing fleas into the home.
Treatment and prevention
For flea elimination programmes to be successful adult fleas must be killed on the pet before they can initiate reproduction and lay eggs. While growth regulators and environmental insecticides are useful at controlling environmental populations no product will kill significant numbers of pupae in the environment. As a result, effective rapid killing of adults before reproduction can take place is required to completely break the reproductive cycle. To achieve this good advice must be given, both in terms or recommending efficacious products and also correct method and frequency of application. Compliance is a pivotal factor and owner preference in terms of use of a spot on preparation, collar or tablet should also be considered. Nurses play a vital role in persuading clients at reception and in clinics of the need for a structured compliant control programme.
The basis of flea control is three fold.
- The use of an effective adulticide — to prevent egg laying and break the reproductive cycle. The product used should be 100% effective, kill adults within 24 hours and be applied frequently enough that this level of efficacy is maintained.
- Effective environmental control — pyrethroids in the environment are useful in reducing numbers of eggs and larvae. Where pet fish and reptiles may be put at risk, or where young children are present in the household, silicone sprays are an alternative. Growth inhibitors such as S-methoprene, pyriproxyfen and lufenuron are also useful. Daily vacuuming of areas where pets spend a lot of time has also been demonstrated to reduce the number of pupae in the environment. Washing of bedding can be helpful, but only if carried out at at least 60 °C.
- Management of client expectation — flea infestations take months to eliminate, even when using effective control measures and if this is not made clear to clients they may become disheartened very quickly.
Questions are often asked by clients about the possibility of resistance to flea treatment products when adults are seen on pets and bites occur after flea treatment products have been administered. Despite numerous large scale studies into the efficacy of flea treatments, there is currently no definitive evidence of flea resistance in the field. Even where resistance genes are known to exist in laboratory strains of flea, fipronil, selamectin and spinosad have all been shown to be highly efficacious at 3 weeks post application (Bass et al, 2004; Dryden et al, 2013).
There are numerous reasons why flea control can break down and resistance perceived to be occurring:
- Not treating all animals in the home — allowing fleas to continue to breed. Ferrets and rabbits also need to be treated for effective cat flea control as well as wildlife and cats visiting the home
- Not treating pets frequently enough — any gap in treatment will allow fleas to breed and infestation to persist
- Not treating the environment — without treatment of the environment some flea infestations will take many months to eliminate (Dryden et al, 2000)
- Fleas other than cat fleas being present — Pulex irritans will also require people living in the house to be treated and poultry fleas will require environmental treatment and removal of any nests that may be a source of infestation
- Lack of management of expectation — heavy infestations of fleas will take at least 3 months to eliminate, even when environmental treatment is used (Dryden et al, 2000). Ironically, elimination will take longer in temperate climates where the reproductive rate is slower and it takes longer for environmental stages to reach adulthood and then be eliminated by adulticides.
If it is assumed flea insecticide resistance is the cause of any single failure of flea control without considering other possible causes, there is then a danger of further client disappointment and frustration when underlying problems and expectation are not addressed.
Toxocara canis and cati
T. canis and T. cati roundworms are found in almost all dogs and cats respectively at some time in their lives. Although dogs may be infected by ingesting embryonated eggs, the most important route of canine infection is transplacental. As a result, prevalence of infection in pups born to untreated dams can reach close to 100%. Dogs may also become infected by trans-mammary infection or consuming paratenic hosts such as rodents. The latter is more important in T. cati transmission where the feline host frequently hunts and trans-placental transmission does not occur. Trans-mammary infection ensures a high prevalence of puppies and kittens shedding large numbers of eggs without anthelmintic intervention. Although immunity leads to a significant reduction in prevalence of patent infection from 6 months of age, arrested larval stages and reinfection lead to intermittent shedding of eggs.
The eggs when first shed are unembryonated (Figure 6) and are not infective. Progression to the infective embryonated l3 stage is required for infection and takes 3–7 weeks so fresh faeces do not present a zoonotic risk. Once Toxocara spp. eggs embryonate, they represent a zoonotic risk that can lead to debilitating illness (visceral larval migrans), ocular damage (ocular larval migrans), neurological defects (neurological larval migrans) and increased risks of chronic conditions such as asthma, epilepsy and dermatitis. 2–4 year olds are most commonly affected, although clinical disease in adults does occur (Overgaauw and Van Knapen, 2013).
Although it has been proposed that people can be infected by eating the undercooked meat of paratenic hosts such as wild game, the most common route of human infection is by the ingestion of embryonated eggs. It was originally thought that T. canis alone was the source of human infection by this route but there is now strong evidence to suggest that T. cati is significantly involved as well (Fisher, 2003). Zoonotic infection through oral ingestion of embryonated eggs can occur through accidental or deliberate consumption of contaminated soil (geophagia), fruit, vegetables and toys (pica) or through transfer of eggs from the coats of pets. The basis of toxocarosis control is therefore reducing environmental contamination with eggs and reducing exposure.
Control of human toxocarosis
Although the incidence of clinical toxocarosis is low there is potential for serious consequences of infection and the tragedy when these complications occur, especially in children, is that with adequate control measures infection may be completely preventable. Control strategies for human toxocarosis involve a combination of the following measures.
- Regular deworming of cats and dogs — puppies and kittens provide the largest source of potential infection. Treatment of puppies should start at 2 weeks of age, repeated at 2 weekly intervals until 2 weeks post weaning, and then monthly until 6 months old. Kittens should be treated in the same way but the first treatment can be given at 3 weeks old as there is no trans-placental transmission. It has been demonstrated that use of an effective anthelmintic every 3 months significantly reduces Toxocara spp. ova shedding and there is no evidence that less frequent deworming frequencies will have any effect on egg output. Therefore, this frequency should be a minimum recommendation in dogs and cats. Use of a monthly anthelmintic will reduce egg output by over 90% and whether this is necessary will depend on the pet's lifestyle. Those pets hunting or in contact with young children or immune suppressed individuals should be dewormed monthly.
- Minimising environmental contamination with cat and dog faeces — UK county councils are taking this threat increasingly seriously and have instituted a number of measures including clearly visible convenient disposal bins (Figure 7), imposing fines for dog fouling, DNA testing, banning dogs from children's playgrounds and sports fields as well as covering sand pits when not in use to try and prevent faecal contamination from cats.
- Good hand hygiene — washing hands after petting or handling cats and dogs and after playing in soil/sandpits is essential to help block transmission of a wide range of zoonotic pathogens.
- Washing fruit and vegetables — intended for raw consumption in case of faecal contamination.

Angiostrongylus vasorum
Angiostrongylus vasorum has been spreading north throughout the UK over the past decade. In addition to increased movement of dogs around the country and increasing fox numbers, this is likely to be due at least in part to climate change. The climate in the UK in recent years has favoured the survival and propagation of slugs with a wetter, milder climate not only increasing their numbers, but also their activity, with the likelihood that they will be eaten. Environmental conditions in which A. vasorum larvae are likely to survive for longer and be more active mean that snails and slugs are also more likely to be exposed to L1 larvae passed in faeces and subsequently become infected.
Increased reporting of cases has been seen in domestic dogs, with 20% of practices across the country having seen at least one case over a 12 month period (Kirk et al, 2014). Post mortem surveys carried out on foxes in 2005 and in 2014 which act as wildlife reservoirs showed overall prevalence of infection rose from 7 to 18% in foxes during this period and extended to regions previously clear of infection such as Northern England and Scotland. The spread of A. vasorum is therefore genuine but not uniform, with focal areas of very high prevalence and other areas remaining free of infection. Case reporting sites such as the IDEXX map have been useful in mapping areas of high prevalence and the introduction of A. vasorum into new areas. Reporting of cases is only voluntary however, and distribution of infection very fluid.
This makes awareness essential of potential A. vasorum cases in areas where the parasite was not previously endemic.
Prevention
Dogs at high risk of exposure should be placed on a routine preventative treatment of a licensed moxidectin spot on solution or a licensed oral milbemycin oxime product. These includes dogs living in known highly endemic areas, young dogs, those previously exposed to infection, eating slugs and snails as well as those eating grass which may contain small slugs. In areas where endemic status is less certain, then testing of dogs prior to surgery and suspected cases such as coughing dogs and those with unexplained coagulopathies will rapidly build up a picture of whether A. vasorum is endemic in an area and whether routine prophylaxis for dogs is required. This data accumulation is vital if risk-based advice is going to be given. Screening suspected cases has been made easier by AngioDetect™ (Idexx Laboratories, USA), a point-of-care blood test which detects circulating A. vasorum antigen in the blood. It has a reported sensitivity of 84.6% and specificity of 100% (Schnyder et al, 2014). This test allows for more rapid diagnosis in a clinical setting and also allows many dogs with clinical signs compatible with A. vasorum infection to be tested relatively economically and rapidly. This in turn allows a picture within practices to be built up as to whether A. vasorum is present in the local area. This picture needs to constantly updated, however, as the regional prevalence of A. vasorum can be very fluid.
Ixodes ticks and Lyme disease
Lyme disease caused by spirochete bacteria of the Borrelia burgdorferi complex is transmitted by Ixodes spp. ticks and although it has been reported in a wide variety of mammals, canines and humans seem relatively more susceptible to disease.
The reported incidence of Lyme disease in people is increasing, but comparable data in cats and dogs is lacking and there is a significant gap in our understanding of canine and feline Lyme disease. There has only been a marginal increase in Borrelia prevalence in ticks on dogs from 2.3% to 2.37% in the last 7 years, but the overall number of ticks on dogs in the UK has increased (Abdullah et al, 2016). This suggests that dogs are being exposed to increased numbers of Borrelia infected ticks, but research is still required to establish how this is translating into prevalence of Borrelia spp. infection and incidence of Lyme disease in UK dogs. 1.8% of ticks on cats were also found to be infected (Davies et al, 2017).
Clinical signs
Dogs may be subclinical or present primarily with acute or sub-acute arthritis in one or more joints with associated lameness, joint swelling and heat. Other acute signs may then follow including fever, anorexia, lethargy and lymphadenopathy. The acute form is often transient with relapses occurring. The common primary human clinical presentation of a circular skin rash known as erythema migrans is not recognised in dogs. Chronic disease is often described in human infection but is less commonly seen in canines. When it does occur in dogs it tends to occur when acute cases have not been treated and consists of a non-erosive polyarthritis. Protein losing nephropathy can be a sign of renal disease in chronic Lyme disease patients. This should not be confused with the syndrome Lyme nephritis which is currently limited to North America.
Diagnosis and treatment
Lyme disease should be considered as a differential diagnosis in pets presenting with any of the clinical signs described, especially those with a history of tick exposure. A number of tests are available that may be considered in Lyme disease diagnosis:
- Tissue or joint fluid culture — this is the gold standard for diagnosis but takes 6–8 weeks with a low sensitivity. As a result, it is impractical in the clinical setting
- Serology — enzyme linked immunosorbent assays (ELISA) or immunofluorescence antibody testing (IFAT) are commercially available. Detection of antibodies in blood or synovial fluid samples is a highly sensitive and specific method of diagnosis. Results confirm exposure to Borrelia spp. exposure and correlate well with clinical infection
- Polymerase chain reaction (PCR) — this is a highly specific test and a sensitive method of diagnosis if synovial membrane or skin samples from the site of the original tick bite are used. PCR from blood or joint fluid carries low sensitivity.
Early intervention with antibiotics is effective in reducing spirochete numbers and improving signs of arthritis. Doxycycline at 10 mg/kg per os once daily remains the mainstay of treatment although amoxicillin may be used as an alternative. In the majority of cases 4 weeks of treatment will be sufficient to clear infection.
Imported novel parasites
A variety of exotic parasites and their vectors have been diagnosed in travelled UK cats and dogs over the past 2 years.
- Ticks and tick-borne diseases — pockets of the tick Dermacentor reticulatus have been long established in the UK in West Wales, Devon, Essex and London. D. reticulatus is the vector for Babesia canis, a cause of potentially life-threatening anaemia in dogs, and while B. canis had been absent from the UK, these ticks presented an opportunity for B. canis to become endemic if introduced through travelling dogs returning from abroad or pet imports that may be infected or carrying infected ticks. Since the establishment of Public Health England's (PHE) Tick Surveillance Scheme (TSS) in 2005 there have been 27 records of D. reticulatus that have not been associated with overseas travel. Seven of these records were submitted during 2016 which represents the highest number of records received in a single year to date (Medlock et al, 2017). This combination of factors has led to an endemic foci of B. canis infection establishing in Harlow, Essex, with B. canis being confirmed in local Dermacentor ticks and in untravelled dogs (Phipps et al, 2016). Further untravelled case were confirmed in Romford in 2016 and Ware in 2017. The potential for spread has also been demonstrated by B. canis being confirmed in a tick from one of the endemic foci in Wales (De Marco et al, 2017). Rhipcephalus sanguineus capable of carrying a wide range of tick-borne diseases pathogenic to dogs including Ehrlichia canis are being seen regularly on imported and travelled dogs. The Big Tick Project examining dogs across the UK for ticks, found that of all the dogs that had travelled in the 2 weeks prior to their inclusion in the study, 30.2% were infested with Rhipicephalus sanguineus (Abdullah et al, 2016). The European Scientific Counsel for Companion Animal Parasites (ESCCAP) UK & Ireland has also seen an increased number of E. canis cases reported in travelled dogs in 2017. Although it is unlikely that R. sanguineus would currently establish outdoor endemic populations in the UK, it can complete its life cycle in 3 months, which has allowed it to infest UK homes in a similar way to fleas. This is a concern, as these ticks may carry zoonotic pathogens such as Rickettsia conorii. Records of R. sanguineus received by the TSS are increasing each year and in the past 2 years there have been two confirmed house infestations (Hansford et al, 2018). A Hyalomma lusitanicum tick was also recently found on a dog in the UK that had returned from Portugal. This species of tick is a potential vector of Crimean Congo haemorrhagic fever virus (CCHFV) which is highly pathogenic to people (Hansford et al, 2016).
- Leishmania infantum — ESCCAP UK & Ireland has seen an increased number of L. infantum cases reported in travelled dogs in 2017 and 2018.
- Thellazia callipaeda — T. callipaeda is a vector-borne eye worm with hosts including dogs, cats and humans. The first confirmed cases in the UK were recently recorded in dogs imported from Romania, Italy and France. Should T. callipaeda be introduced to the UK, its primary fruit fly vector P. variegate has been recorded in the UK with conditions favourable for spread (Graham-Brown et al, 2017).
- Dirofilaria repens — the first cases of the skin filarial nematode D. repens were recently confirmed in UK dogs imported from Corfu and Romania (Agapito et al, 2018). While not highly pathogenic, D. repens is a zoonosis and is transmitted by mosquitoes endemic in the UK. D. repens infection in dogs appears to be spreading across Europe, with a corresponding increase in zoonotic cases. Infected dogs act as reservoirs of infection for the mosquito vector, which is present in the UK. If infected dogs continue to enter the UK and are not treated quickly, there is the possibility for UK mosquito populations to be exposed to the parasite and for the disease to become endemic.
- Linguatula serrata — a number of nasal pentastomid L. serrata (tongue worm) cases have been seen in the UK in dogs imported from Romania. Infection is acquired from the consumption of raw meat and offal in endemic countries. Infections in dogs are often subclinical, but may present with nasal discharge, sneezing and epistaxis. L. serrata is a zoonotic infection with humans acting as intermediate as well as definitive hosts, and may therefore become infected through ingestion of eggs from nasal secretions and faeces.
Parasite control in imported pets
ESCCAP UK & Ireland recommend four key steps when dealing with all imported or travelled pets arriving in the UK:
- Check for ticks and subsequent identification of any found — identification of ticks allows the introduction and distribution of exotic tick species to be monitored in the UK, but also indicates which tick-borne diseases the imported pet may have potentially been exposed to. Bristol University has a tick identification key to aid with identification (http://www.bristoluniversitytickid.uk/) or they can be sent to the PHE TSS for identification (https://www.gov.uk/guidance/tick-surveillance-scheme).
- Treat dogs again with praziquantel within 30 days of return to the UK and treat for ticks if treatment is not already in place — this will ensure that Echinococcus multilocularis is eliminated from imported pets, whenever the timing of their compulsory treatment, or if there is doubt about it having been administered correctly/at all. Tick treatment will increase the likelihood of attached ticks being killed if they are missed on examination. Ticks are easily missed in large or long-coated breeds and in cats and dogs that are reluctant to be examined.
- Recognise clinical signs relevant to diseases in the countries visited or country of origin — a thorough and comprehensive clinical examination of imported pets will identify adverse clinical signs and these can then be compared with parasitic diseases in the countries that the pet has visited.
- Screening for Leishmania spp. and exotic tick-borne diseases in imported dogs — both Leishmania infantum and tick-borne diseases can have long incubation periods and carrier states. Infection can also be lifelong and in some cases can carry a poor prognosis. Screening for these parasites will lead to early diagnosis, preparing the owner for what could be a lifetime of potential treatment, any associated zoonotic risk and limiting wider spread through effective tick control.
Conclusions
Nurses are an integral part of the practice team, helping to formulate parasite control programmes for clients and giving accurate parasite control advice. Although the number of parasites and products to treat them may seem daunting, it is vital that nurses remain up to date with current parasite distributions and appreciate that these distributions are fluid. Further parasite control information can be obtained, and questions asked on the ESCCAP UK & Ireland website (http://www.esccapuk.org.uk).
KEY POINTS
- Ctenocephalides felis is the most common flea found on cats and dogs and is of veterinary significance as a vector of disease, cause of flea allergic dermatitis and a source of bites and revulsion to owners, making flea control vital.
- Deworming at least 4 times a year is also essential to reduce Toxocara spp. egg shedding.
- Distributions of the parasites of veterinary and human health significance in the UK, such as Angiostrongylus vasorum, ticks and Echinococcus granulosus, are changing rapidly.
- In addition, parasites exotic to the UK are being imported on travelled pets.
- Nurses form a vital part of the practice team when giving parasite control advice and need to remain up to date with parasite distributions and appreciate their fluid nature so accurate parasite risk can be assessed and appropriate advice given.


