In the last decades, an increase in pet ownership of lizards, snakes and chelonians has developed. Despite the controversies currently being debated surrounding the need to balance animal welfare, human health and wellbeing and conservation needs, reptiles remain popular pets, accounting for 1.6% of the estimated 12 million UK homes reporting pet ownership in 2017 (44% of all households) (PFMA, 2017). The abundance of tick species associated with reptiles means that veterinary nurses would benefit from an understanding of the tick life cycle, host preference and attachment sites. In addition, three groups of mites are known to be the main cause of infestations in reptiles in captivity, and play a role in clinical disease. This article, therefore, reviews the major groups of ectoparasites found in confined reptiles, along with their significance and factors contributing to clinical disease, and outlines the treatment options.
There are no reports of the common mammalian ectoparasitic insects, such as fleas and lice, in reptiles, along with clinical symptoms. These insects are therefore not included in this clinical review; the focus will be the arachnid ectoparasites, mites and ticks.
Mites
With most species of mites dwelling on the skin surface, heavy infestations are seldom found in wild reptile specimens because the ecdysis process (skin shedding) provides sporadic removal of the mite population (Davies, 2008). Mites are commonly found on captive reptiles and are feared as pests themselves and as vectors of diseases. Mite infestations are especially common in crowded situations and with poor husbandry (in small terrariums and under sub-optimal housing conditions, too high/low humidity, too low temperature). Mites cause damage to the host when drawing blood, and a heavy load can be life threatening and can cause major damage to the skin and hyperkeratosis (Reichenbach-Klinke, 1977). The number of species that parasitise snakes and lizards can be significant, although mites play a minor role in chelonian diseases. The body regions that are affected vary depending on the species; however, often the protected areas and skin folds are infested, such as the spaces around and along the bottom of the scales, the axilla, the tail base and the environment of the eyes.
In lizards, mites (especially Trombiculidae larvae, rare Pterygosomidae stages) are localised in so-called mite pockets (acarinarium), in small skin folds in the neck, armpit, inguinal region or tail base (Arnold, 1986).
Ophionyssus natricis
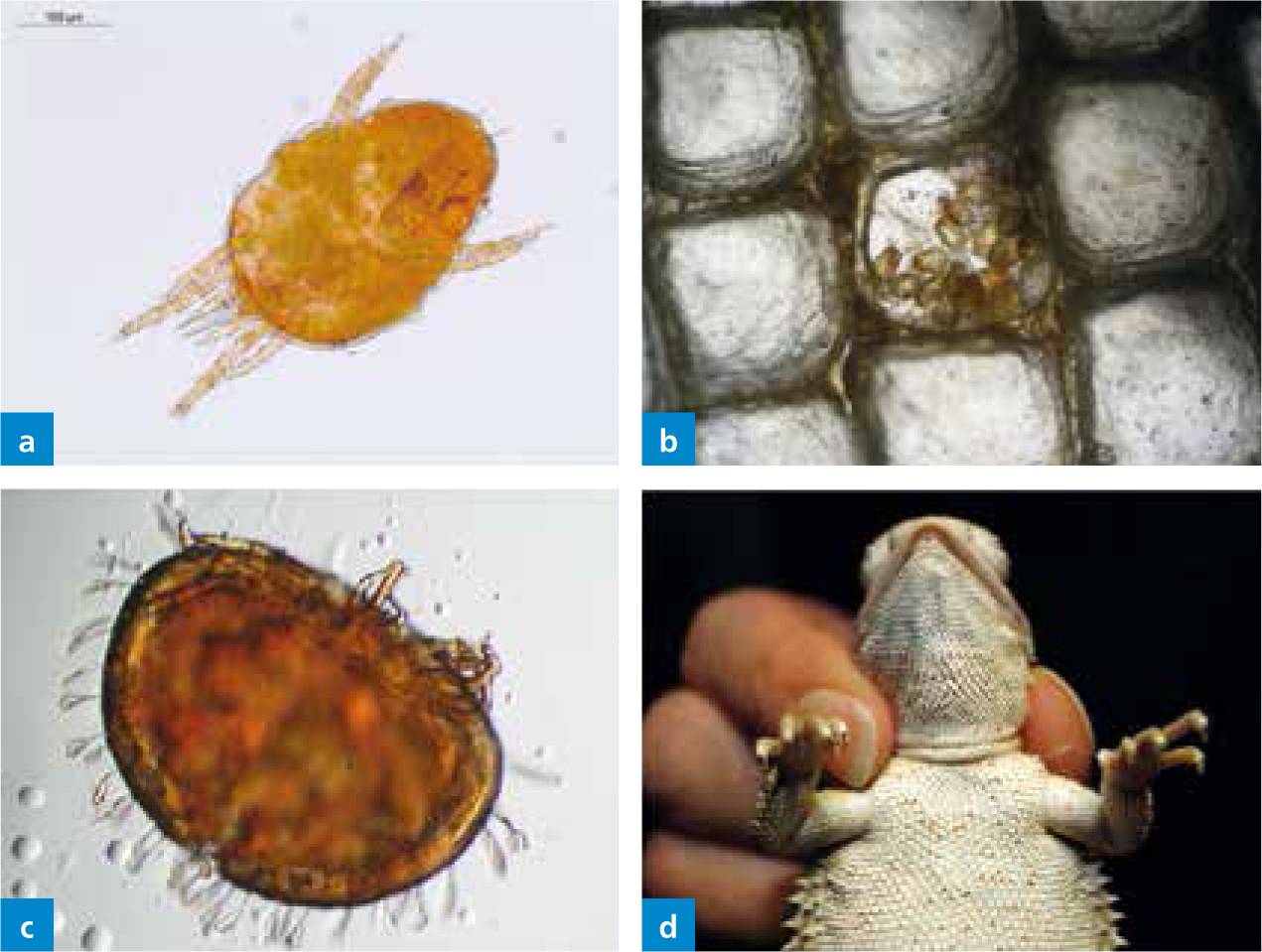
The snake mite (O. natricis; Order Acari; Class Arachnida; Family Macronyssidae), also known as the ‘blood-sucking’ mite, is the most common ectoparasite of snakes worldwide. This is a common blood mesostigmatid mite (De-Nardo and Wozniak, 1997). This mite species can also be found on lizards (e.g. iguanas or bearded dragons) (Beck and Pantchev, 2006), when its preferred host is not available or when a wide variety of reptiles are housed together. Generally, snake mites are not found on turtles. There is currently only one case report documenting O. natricis infestation of Testudo hermanni boettgeri (Wiechert, 2007). It occurred on two juvenile animals, around 6 months old, which were purchased from a pet store along with the furnished terrarium. Later questioning found that the merchant had kept live snakes in the terrarium prior to selling it as a new, unused terrarium. Although the animals appeared to be healthy when purchased, mites were found in the terrarium just 3–4 days after bringing them home. The turtles were extremely irritated and were often seen rubbing the eyes on the forelimbs. On examination in the veterinary practice the first observation was of numerous, small white dots found on the carapace (this was the guanine excretion of the mites) (Figure 1). Many mites were also found on the soft tissues of the body. There were both nymphs (Figure 1b) as well as adults of the snake mite (O. natricis), and most were found around the head and on the neck.

Life cycle of O. natricis
The life cycle of O. natricis (7–16 days) consists of egg, larva, protonymph, deutonymph and adults stages (Camin, 1953) (Figure 1). The primary cage functions as the ideal nest-like environment, and because of the relative short cycle, mite infestation can spread very rapidly among dense reptile populations. The eggs are sticky, pale ovoid structures (400–300 μm) with one of the poles darkening as development progresses. Larvae are small and white, they remain close to the eggs, and they need at least 75% relative humidity to moult to the protonymph stage. In contrast to the larvae, this next stage is a very aggressive blood-feeding stage with well-developed chelicerae, and on contact of a suitable host they will crawl under the scales or around the eyes and start feeding (Figure 1). They then detach from the host and moult to the next stage, the deutonymph, in 12–48 hours. This stage is a non-feeding stage and is rarely found on the host. Adults are dimorphic and feed on blood. They are brownish and have a tapered body towards the caudal end. Each blood meal will result in the production of approximately 20 eggs (Wozniak and DeNardo, 2000). Mites are able to move purposefully, whereby females generally tend to move upwards. This is why many mites are found in the upper half of the terrarium. Mites can escape through ventilation openings and may infest every terrarium in the room within 1 day.
Clinical importance
This mite can be a vector of blood-borne protozoal (e.g. hemogregarines) as well as viral (inclusion body disease (IBD)) pathogens of snakes (Camin, 1948; Chiodini et al, 1983; Schumacher et al, 1994). Lesions caused by these mites could also serve as portals of entry for bacteria like Aeromonas spp. However, further vector competency studies need to be performed to characterise the overall role of this parasite in the transmission of other infectious agents (i.e. filarids). Infestations can cause dehydration, lethargy, growth impairment (Wozniak and DeNardo, 2000) and in high infestations, anaemia and dysecdysis impairments (DeNardo and Wozniak, 1997; Davies, 2008). Individuals affected can be found most of the time in the water to relieve the pruritus caused by the dermatitis, with the skin becoming hyperaemic and oedematous (Wozniak and De-Nardo, 2000). There have been reports of loreal pit inflammations and impactions associated with heavy infestations (Garrett and Harwell, 1991). Histopathologic evaluation of feeding sites shows infiltration with neutrophils, lymphocytes and plasma cells, with multifocal perivascular aggregates of lymphocytes and plasma cells in the adjacent dermis (DeNardo and Wozniak, 1997).
This parasite has zoonotic importance due to the voraciousness of the protonymphs as they can swarm and bite humans in the enclosures, causing papular vesiculo-bullous eruptions in the skin in entire families (Schultz, 1975), and other bite-associated dermatitis (Beck, 1996).
Diagnosis
Regular inspection of the reptile for both the free-living (eggs and deutonymph) as well as the parasitic (protonymph and adults) stages is crucial to maintain control of this mite. During the clinical examination of snakes, close attention to the periocular skin, chin shields and the first two scale-rows is of paramount importance. A useful tool is to swab the sides of the snake with gauze and immediately examine it (Wozniak and DeNardo, 2000). Adult mites measure 0.–1.3 mm, they are pale brown when unfed, but red or black when plethoric. Another useful tool is to bathe the snake regularly in warm water in a white container so the black specks (mites) can be observed floating around (Davies, 2008). Mild infestations can be detected by placing a piece of white blotting paper inside the terrarium below the lid — the black mites are more easily visible on a white background.
Control of mites
Due to the life cycle having free-living and parasitic stages, it is rather difficult to control this mite. Because of this complexity, the best approach to follow is an IPM, integrated control programme, which is composed of isolation, sanitation, topical and systemic acaricides, and habitat-based pesticide applications. Infested animals should be isolated in a quarantine area for 2–4 weeks. Cages with infested snakes should be emptied and thoroughly washed at least twice a week (Pfadt, 1985; Wozniak and DeNardo, 2000).
Pyrethrins and pyrethroids diluted at 0.03 and 0.35%, respectively, have been shown to have a high efficacy against this mite in USA (Mader, 1996). Products should be left on for less than an hour, and care must be taken to thoroughly wash off these products with hot water after applying them to reduce pesticide absorption — repeat every 10–14 days (Sidon et al, 1988). Studies have shown that mite-parasitic mites, such as Phytoseiulus persimilis (Davies, 2008) and Cheyletus eruditus (Schilliger et al, 2013), are a viable and important alternative and option for biological control — these feed on O. natricis.
Two medications have been used successfully and with few side effects in the UK — ivermectin and fipronil. Ivermectin is applied topically or by injection to the reptile, and also used to disinfectant the vivarium; fipronil is applied topically and used to disinfect the surface of the cage. Newspaper is often the best substrate during treatment and should be used to line the vivarium as mites cannot hide and are easy to remove and destroy (Davies, 2008). Currently fipronil (Frontline Spray, Boehringer Ingelheim Animal Health UK Ltd) is the medication of choice for treatment of mites on the animal in Europe. It is gently applied to the entire body (not sprayed). Application to mucous membranes must be strictly avoided. In a group of several animals, each individual should be treated. Treatment with fipronil is usually well tolerated, however there are also reports of side effects in reptiles. Adverse reactions and toxic reactions occurred in young boas and chameleons (Beck and Pantchev 2013). Disinfection of terrarium and furnishings for most ectoparasites can be carried out using fipronil (Frontline Spray). In snakes and lizards, ivermectin can replace fipronil as an alternative and can be used to decontaminate the terrarium (Pasmans et al, 2008). Ivermectin, available as a 1% solution, should be diluted by adding 1 ml (10 mg) to 1 litre of water; this solution is stable for up to 30 days (Kahn, 2005). As an alternative to rubbings, ivermectin can be used as a subcutaneous injection with 0.2 mg/kg bodyweight (BW), which is repeated after 14 days. There is known toxicity for ivermectin in some reptiles, such as chelonians and crocodiles (toxic), it is also problematic with very small lizards, chameleons, indigo snakes and skinks (can also cause skin discolouration at the injection site) (Funk and Diethelm, 2006).
Terrariums should be empty during chemical decontamination, and the treated reptiles as well as the water bowls should be put back into it only after complete drying out and airing. Hot water >60°C also kills mite stages; the addition of detergent is recommended as it reduces the surface tension of the water which leads to the drowning of the mites. This can be used as an alternative to chemical decontamination. Surfaces should be allowed to dry thoroughly. Snake mites do not survive temperatures >45 °C and <5 °C or humidity <20% (Camin, 1953). It is recommended that the whole room should be cleaned thoroughly using a vacuum cleaner in order to remove any migrating mites. Applications, as well as environmental decontamination, can be repeated every 5 days within 3 weeks. 5-day intervals prevent recently hatched larvae from reaching sexual maturity; the period of 21 days corresponds to the lifetime of adult mites. Additional tips for terrarium decontamination are given by Schneller and Pantchev (2008).
Supportive measurements can be applied after serious skin damage in iguanas or snakes as, e.g. injections (intramuscular (IM)) of vitamin A (1000–5000 IU/kg every 7–10 days up to 4 treatments); for giant snakes a knife tip of vitamin C dissolved in some water and entered by gavage is also beneficial (Kölle, 2000).
Practical advice
O. natricis is a very important ectoparasite of captive reptiles. It can cause and transmit serious clinical disease. Infestations are very common and difficult to eradicate. Different individual treatments or in combination are available. It is crucial to have a sound knowledge of the biology of this parasite when implementing control strategies. Eliminating contact between mite-free snakes and reptiles of undetermined status is a key factor in the maintenance of mite-free animals. All newly acquired snakes ought to be placed in quarantine for a minimum of 90 days before moving them into the mite-free collection (for general quarantine issues see Pasmans et al, 2008).
Pterygosomid mites (Trombidiformes; Pterygosomatidae)
Pterygosomid mites include Pterygosoma, Hirstiella, Zonurobia, Geckobia and others.
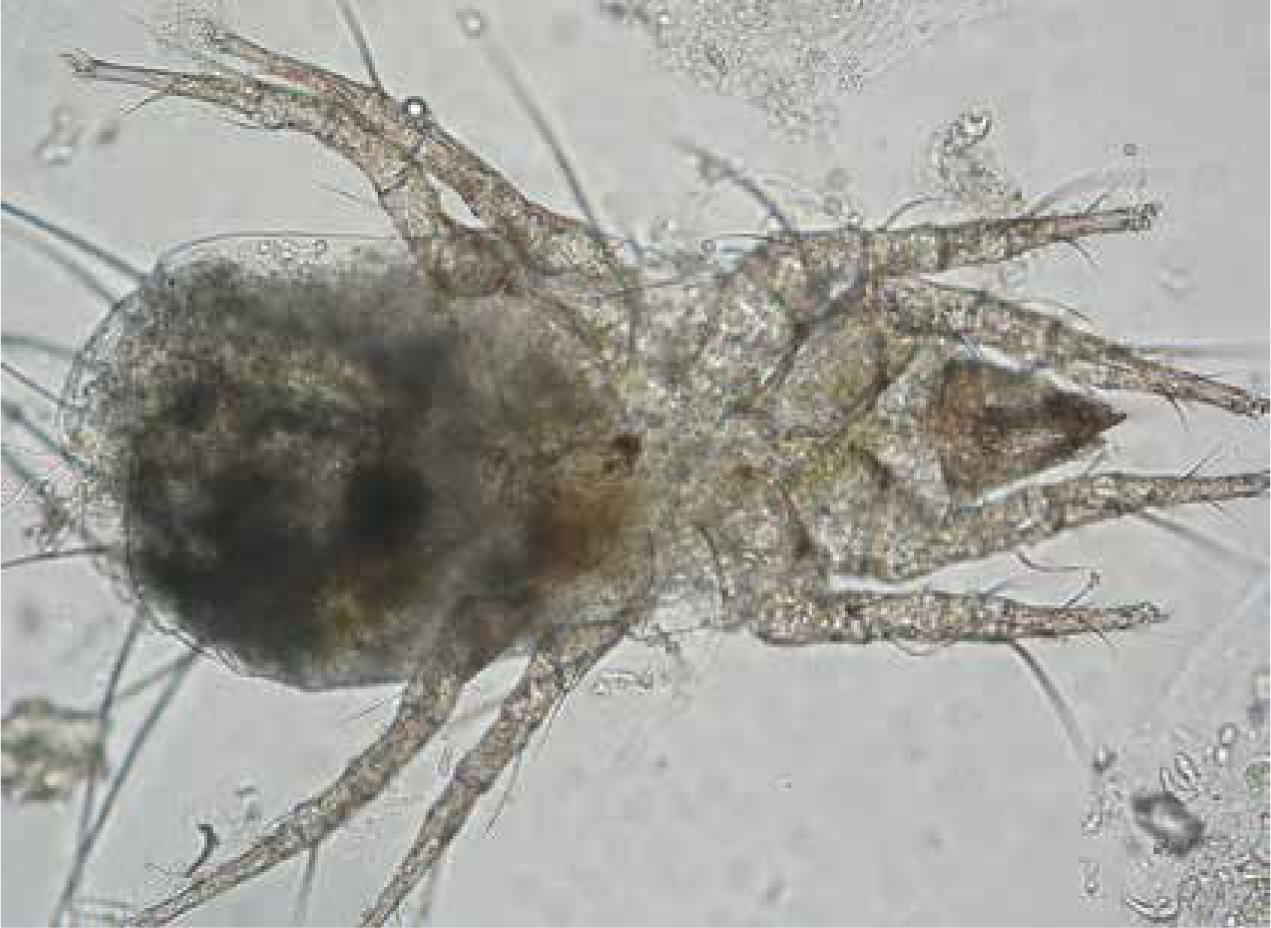
An important differential diagnosis for snake mites includes pterygosomid mites, mainly infecting different lizards. These mites are easily differentiated based on their characteristic appearance (orange to red) (Figure 2); they do not show a zoonotic potential. The orange-coloured pterygosomid mites are well adapted to ectoparasitic life. The body is almost always wider than long, and some of the bristles are remodeled to broad, spatula-shaped, often whitish structures (Figure 2). They are found on lizards of the families Agamidae, Gerrhosauridae, Cordylidae, Gekkonidae, Iguanidae and Tropiduridae. Predilection sites for these ectoparasites are the soft skin at the base of extremities, interdigital skin und underneath scale rims (Figure 2). These mites are usually distributed regularly throughout the body and rarely in so-called mite pockets (see Trombuculidae) (Schmäschke et al, 1997; Bertrand and Modry, 2004; Delfino et al, 2011). Pterygosomids are haematophagous (blood sucking), and the entire development takes 6 to 12 weeks. They rarely multiply in captivity. More indepth information on their development is provided by Schmäschke et al (1997).
Clinical importance
As with many other ectoparasites, they can cause dermatitis (dark-coloured plaques, erythema, swelling, ulcers), and hence the name ‘black skin disease’ is commonly used. In heavy infestations dysecdysis, anaemia and debilitation are common (Mader et al, 1986; Farmaki et al, 2013). These mites can also play a role in the transmission of blood parasites (Schellackia or Hepatozoon).
Diagnosis
Diagnosis is based on the identification of the mites as ‘red spots’ — sometimes they can appear as brown or orange, but this depends on the degree of blood engorgement (Farmaki et al, 2013).
Control
A number of different treatments have been used on lizards infested with this mite. These include immersion in tepid water, whole body application of olive oil, organophosphates, carbamates, pyrethrin or pyrethroid sprays, shampoos and ivermectin injections. However, in terms of safety and efficacy, most of them seem to be toxic and with a low efficacy in some lizard species (Mader et al, 1986; Farmaki et al, 2013). Recently, a topical 0.25% fipronil solution administered with cotton pads showed a significant decrease of mite load following a 7-day course, compared with 2 days post-treatment (Farmaki et al, 2013). This difference in mite reduction could be explained by the slow onset of activity of this medication, or by the movement of the moribund mites which had not been detected before because they were hiding under the scales. Importantly, owner consent should be obtained before treatment as this is an extra-label use. This should always be followed with appropriate environmental cleaning procedures (Farmaki et al, 2013). Control measures are the same as for the snake mite (O. natricis).
Harvest mites/’Chiggers’ (Trombiculidae)
Out of approximately 1500 species within this group, 50 live as parasites only during their larval stage. The most commonly known mite of this group is Neotrombicula autumnalis (Shaw, 1790); known as the harvest mite or autumn chigger, it is also a common seasonal parasite of dogs and cats. Interestingly, the larval stage is the only parasitic stage, whereas the nymphs and adults are free-living predators and primarily feed on small arthropods and their eggs (Harwood and James, 1979).
Example of trombiculid life cycle based on the chigger mite
Eggs are laid in the soil or ground debris. After a 6-day incubation period, a non-feeding prelarval stage hatches, after another 6 days the parasitic larvae develop and they feed on mammals, birds, reptiles and man. Six-legged larvae are 200 to 400 μm in diameter, with the body more or less rounded, with single dorsal scutum and four to six setae (hairs) (Figure 3). They feed primarily on partially digested skin cells and lymph dissolved by their salivary secretions, not on blood. They usually only stay attached for few days, however the host reaction and irritation may persist for several days. After feeding for 5–6 days, the pletoric larvae drop off, and transition into a quiescent prenymphal stage. The adults are about 1000 μm long, may be oval to figure-eight-shaped. They are covered with tiny, dense hairs giving them a velvet-like appearance. The colour of the adult is usually bright red (Harwood and James, 1979).

Clinical importance
Irritation from the chiggers or self-trauma (or both) produces marked oedema and urinary retention. Necrosis and lichenification can also be seen due to high infestations. In lizards in captivity (wild-caught) and free-living lizards trombiculid mites can be localised in mite pockets. At seasonal occurrence they can cause severe lesions. These mite pockets are small folds of skin in the cervical, axillary and inguinal region or tail base (Figure 4) (Arnold 1986; Bertrand and Modry 2004; Delfino et al, 2011). These structures can be considered as a possible defence strategy of the lizard to reduce lesions by restricting the mites to these body regions. There are at least five lizard families (Iguanidae, Chamaeleonidae, Gekkonidae, Lacertidae, Scincidae) with mite pockets already described, found in warm and not completely dry regions. These structures are present in hatchlings, so they arise not only in response to the mite infestation. Characteristically, the epidermis is hyperplastic and resilient, and the lesions induced by the mites are quickly repaired. The dermis in mite pockets also contains a high concentration of immune cells.

Diagnosis
Diagnosis is based on the presence of an orange crusting dermatosis, identification of the typical larvae on skin scrapings and histopathological findings.
Control
If such mites are present on an animal they can be treated like pterygosomid mites. As only the larval stage is parasitic, this infestation is self-limiting and will not affect indoor animals.
Storage mites as pseudoparasites
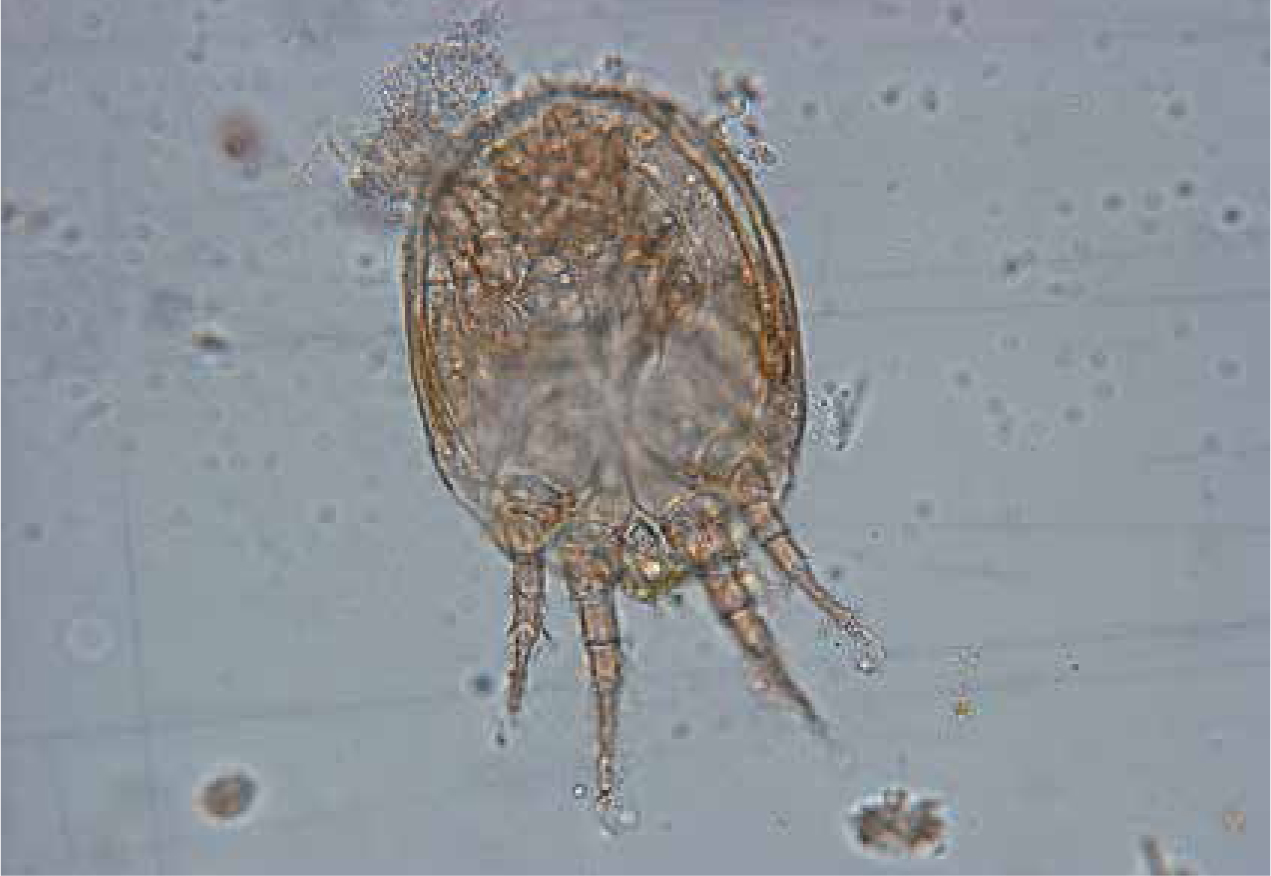
Very often (up to 19% depending on the lizard family), storage mites can be found transiting in the faeces, particularly in insectivorous lizards (Pantchev, 2009). These findings may indicate infestation of the food with mold mites. Occasionally, these mites may also be found in necrotic material from tortoise carapace mimicking a true parasitosis (Figure 5); however, they are not the primary cause of disease, but are present in already damaged and softened carapace tissue, which serves as a food source for the mites. Husbandry conditions would need to be evaluated as a primary cause, for example tortoises kept in humid conditions that prefer arid conditions in the wild. Alongside topical treatment (ablation of necrotic material, washes with chlorhexidine solution), the correction of husbandry conditions is recommended. Sometimes there are also fungal infections present, which should be treated specifically. Many storage mites are able to introduce an additional developmental stage in unfavorable living conditions, which can be occasionally found in reptile faeces or on rare location on reptiles (Figure 6). This so-called deutonymph stage (hypopus) appears between protonymph and tritonymph stages and allows survival in adverse living conditions in the habitat.
Ticks: hard and soft ticks
Imported reptiles that are captive bred are rarely infested with ticks, but many animals destined for the European pet trade originate from tropical countries and are wild-caught (Bush et al, 2014; Robinson et al, 2015). Moreover, wildlife (green pythons, various monitor lizards) laundering through breeding farms, as in the case of Indonesia, exists (Lyons et al, 2011; Öfner et al, 2012). The RSPCA report that between 2000 and 2008, there was a 79% rise in the number of wild-caught reptiles being imported into the EU, and estimate that between 5.9 and 9.8 million live reptiles were imported into the EU in 2009 (RSPCA, 2011). Veterinary professionals, when examining the pet reptile, should be aware that the risk of tick infestation is greater in legally wild-caught reptiles, but that illegal trade routes are also a source of pet acquisition.
Reptiles are natural hosts for a range of ticks from the genus Argas, Ornithodoros (both belonging to the soft ticks, Argasidae) and Amblyomma, Dermacentor, Haemaphysalis, Hyalomma and Ixodes (which are hard ticks, Ixodidae).
Soft-bodied ticks, which have a higher classification evolutionarily, are very rarely found on reptiles (Hnizdo and Pantchev 2011). One reason might be the differing biology. In soft-bodied ticks only the six-legged larvae (very small — they lie under the scales) remain attached to the host for a long time. The other stages (nymphs and adults) feed generally at night for 10–30 minutes; for this reason, they would remain unnoticed in the terrarium. Occasionally Ornithodorus talaje, a species of soft tick, may be imported unnoticed from South or Central America, and can cause an epidemic.
Hard ticks frequently parasitise reptiles, particularly terrestrial chelonians, snakes and lizards in tropical, subtropical and warm temperate regions (Cumming, 1998; Barnard and Durden, 2000; Hernandez-Divers, 2006) (Figure 7). The importation of exotic (non-native) ticks in the USA has been well documented (Burridge, 2005). Between 1962 and 2001, a total of 29 species were introduced via the importation of reptiles, of which the majority were from the genus Amblyomma and (formerly) Aponomma. The tick–host associations, geographical distribution, life cycle and vector potential of these imported species are well described in Burridge and Simmons (2003), and highlight the variety of reptile hosts and tick species that are transported globally. Across Europe, country-specific data on the importation of ticks is more limited, but there is increasing evidence that they are being introduced via the international trade in reptiles. A recent review concluded that of reptile ticks imported into Europe, the majority belong to the genus Amblyomma and Hyalomma (Milhaca, 2015). In a survey of reptiles imported to the UK via the animal reception centre (ARC) at Heathrow airport, five species of exotic tick were recorded: Aponomma (Amblyomma) exornatum, Ap. (Amblyomma) latum, Amblyomma dissimile, Am. nuttalli (Figure 8) and Am. rotundatum, associated with white-throated monitor lizards, ball pythons, caimans, bell's hingeback tortoises and cane toads respectively (Pietzsch et al, 2005). Of note, these authors also report that occasional consignments imported via legal channels, initially deemed tick free, were subsequently found to be infested on arrival at ARC, most likely due to undetected eggs hatching following exportation. Another possibility is laundering of wild-caught animals through breeding farms (see above). Similarly, a survey of reptiles imported to a live animal wholesaler company in Poland detected six species of ticks, comprising 2104 individual tick specimens: Amblyomma exornatum, Am. flavomaculatum, Am. latum, Am. nuttalli, Am. quadricavum, Am. transversal, Am. varanense and Hyalomma aegyptium. The majority were from pythons and monitor lizards (Nowak, 2010a).


As with endemic (native) ticks, the general life cycle of the tick consists of three life stages, a larval, nymphal and adult stage, each feeding for a period on a host, dropping off the host to moult in the environment, and emerging into the subsequent stage. In some tick species, the engorged larvae will remain on the host, moult into nymphs and begin feeding again. Adult females, once fully engorged and mated, will drop off the host to lay eggs, the number varying between one species and another. The life cycle processes are strongly influenced by climatic conditions, predominantly temperature and humidity. Feeding ticks remain attached for long periods — several days depending on the life stage in mammals. In reptiles, the time from the attachment to the body to the complete engorgement of adult females may take weeks to months (Barnard and Durden, 2000). Hence, ticks may be transported along with their hosts to new international destinations.
The description of specific tick species biology is beyond the scope of this article, and is well described elsewhere (Barnard and Durden, 2000; Burridge, 2001; Walker and Bouattour, 2003).
Tick species vary in their host specificity, some feeding exclusively on one host species, others exhibiting a two or three host life cycle. The host on which each stage feeds can be exclusively reptiles, or often the immature stages will feed on mammals or birds. Knowing the body site location at which ticks prefer to feed will help veterinary professionals identify areas on which to focus clinical examinations, however, the site of tick attachment varies between the different tick species. For example, Amblyomma marmoreum feeds exclusively on tortoises, particularly leopard tortoises, attaching to the softer body parts around the legs and tail. Amblyomma exornatum feeds on varanid lizards, frequently found in the nostrils of these reptiles (AFRIVIP, 2018).
Clinical importance
The significance of ticks is their ability to transmit viruses, bacteria and protozoans to the host animal (Barnard and Durden 2000). The bacterial aetiology of the human pathogen Q-Fever for example was isolated from the African, reptile-specific Aponomma spp.; the hard-bodied tick Hyalomma aegyptium is known to be the carrier of the haemogregarine Hemolivia mauritanica, whose gametocytes are found in the erythrocytes of tortoises such as Testudo graeca and T. marginata. Hard-bodied ticks from the genus Amblyomma carry Ehrlichia (Cowdria) ruminantium (an obligate intracellular, parasitising bacteria) — the causative agent of heartwater disease in ruminants. Since it was shown that E. ruminantum was in Am. sparsum found on Stigmochelys pardalis imported into Florida from Zambia, these turtles can no longer be imported into the USA. In a study in Great Britain where 39 ticks from ten imported reptiles were studied, polymerase chain reaction (PCR) and subsequent sequencing proved that all individuals from the genus Aponomma had apicomplexan protozoa (Hepatozoon spp.) (Kenny et al, 2004). In three ticks from the genus Aponomma from Africa it was possible to identify ‘Ehrlichia’-like organisms.
Most Amblyomma species cause painful skin lesions including inflammation and ulcers (Burridge, 2005) (Figure 7). The blood loss from the feeding ticks is generally minimal; the long-term blood sucking is balanced by increased haematopoiesis. This compensation mechanism can become unbalanced in a massive infestation on a small body. The unnoticed multiplication of soft-shelled ticks in a terrarium can lead to anaemia and even death.
Diagnosis
The inspection of reptiles imported by a wholesaler in Poland detected ticks on various locations of the monitor lizards and pythons. The majority of ticks removed from monitor lizards were located on the limbs, primarily around and between the toes, followed by the trunk and head, but numerous ticks were also removed from the nasal passages and ears (Nowak, 2010b). Of the pythons examined, the majority of ticks were removed from the back and abdominal side of the trunk, and to a lesser extent from the cloaca, around the eyes and the nasal openings (Nowak, 2010b).
Ticks are known to aggregate in particular body sites due to the release of pheromones that are important in mating, and because ticks seek areas of the body where the skin is thinner and therefore access to a blood meal is easier. These sites are enabling ticks to remain attached and achieve full engorgement. It may be useful for the veterinary professional to ascertain when and where the pet reptile was purchased to determine the likelihood of infestation with particular species of ticks.
Control
Whenever possible, all ticks should be mechanically removed (Figure 9). The ticks should be grasped with special tick-forceps or tweezers as close as possible directly against the scales to include the mouthparts; then pulled up firmly and in one direction. If the movement is not firm, the mouthparts may break off and remain in the skin. This may cause formation of an abscess, especially in reptiles kept in humid environments. Once the tick has been removed the bite location should be cleaned with an antiseptic solution, such as alcohol. Never should one attempt to kill a tick before removal using oil, alcohol, glue, nail polish or similar products. This could cause infectious agents from the tick's intestine to migrate into the host to inoculate it. In case of a heavy load, when it is not possible to mechanically remove all the ticks, or in attempts to maintain collections free of ticks, tick treatments can be used. The affected reptiles can be treated, analogous to a mite infestation, with fipronil application (it kills ticks within 24–48 hours of contact; see mite section for restrictions).

In the USA, there is a product licensed for use in reptiles (permethrin 0.5%: only in the product Proventa-amite spray; Pro Products Mahopac, NY). It was tested as a direct treatment to control tick infestations on African spurred tortoises (Geochelone sulcata) (Burridge, 2005). For a weight above 0.91 kg, two spray bursts (1 second duration) into each leg opening from 30–38 cm distance (the eyes are protected at the front), and below 0.91 kg one spray burst (also 1 second duration) was used. Permethrin is a very fast-acting contact acaricide with a rapid onset knock-down effect against arthropods. Treatment of snakes and lizards should only be done indirectly with this product (Burridge, 2005). In these cases, animals and the water bowls should be removed from the terrariums and the empty cages sprayed from 30–38 cm (1 second per 930 cm2). After complete drying-out and thorough ventilation, the snakes/lizards and water bowls can be returned. In attempting to eliminate ticks from a collection, a plan such as that proposed by Burridge (2005) may be helpful.
Practical advice
Pet reptiles need to be thoroughly examined for ticks and any found removed with tweezers or a tick removal tool. Any ticks found should be removed and sent to parasitologists for identification to determine the vector status of the tick species and determine any potential public health risks. Owners should be reminded to check daily for ticks as immature stages might be difficult to detect due to their small size and their ability to attach under the host scales. The vivarium should be thoroughly cleaned and disinfected to remove any ticks or eggs that may be present.
Although it is unlikely that most reptile ticks will become established in the outdoor environment if accidentally released due to unfavourable climatic conditions, there is a possibility of establishment in some vivarium, if the tick species environmental and host requirements are met.
While awareness of ticks on companion animals and the need for their control has been well documented (Jimenez Castro and Fisher, 2013; Richmond et al, 2017), no reports were found on the occurrence of ticks on reptiles seen in veterinary practice. However, the increased popularity of exotic pets requires the veterinary professional to have a broader knowledge of ticks and an awareness of the requirement to consider ticks in the health assessment of reptiles.
Conclusion
The role of the veterinary nurse is crucial in educating the owners about parasites that may present on reptiles, and on ways to control and treat them. They are often the first and last person with veterinary training to interact with the owners.
KEY POINTS
- Pet reptile ownership has increased substantially over the last few years, with 1.6% of the 12 million homes in the UK that reported having a pet, having at least one reptile.
- The snake mite, Ophionyssus natricis, is the most common ectoparasite of snakes worldwide.
- These ectoparasites can be zoonotic.
- Reptiles are natural hosts for a range of hard ticks and occasionally soft ticks.
- Veterinary nurses are crucial in educating the owners about parasites that may present on reptiles, as well as control and treatment approaches.


