The first article in this series looked at initial wound assessment, preparation and wound lavage (Aldridge, 2013). The aim of wound lavage is to reduce bacterial contamination, and to help remove foreign material from the wound bed, both of which are common impediments to wound healing (Franz et al, 2008; Gregory, 2009). This article will discuss the next step in management of traumatic wounds: debridement.
Aims of debridement
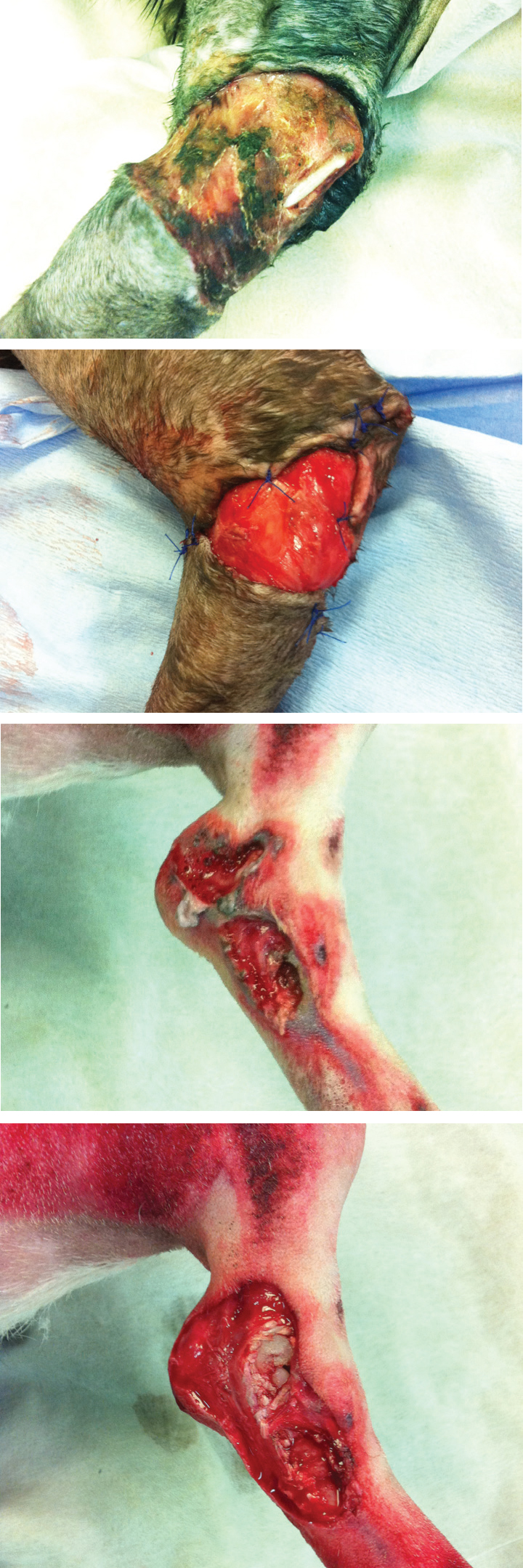
Debridement is defined at the removal of damaged tissue or foreign objects from a wound (Figure 1). Necrotic tissue will delay wound healing and increase the risk of wound breakdown by acting as a nidus for infection. Necrotic tissue also slows wound healing by obstructing re-epithelialisation and wound contraction (Hart, 2002).
Traumatic wounds can be managed by primary closure, delayed primary closure or secondary closure; or they may be left to heal on their own by secondary intention (see Table 1 for wound closure options). The most important factors in deciding whether, and when, to close a wound are the degree of contamination (bacteria and foreign material), and necrotic tissue present (Bellah and Williams, 2009). A combination of lavage with debridement techniques enables the reduction of the level of contamination and the presence of necrotic tissue to a point where the closure technique chosen can be safely carried out. This may not be possible in a single procedure; wounds that have extensive contamination and ischaemia may require repeated debridement, and the use of more than one debridement technique, before closure can be considered. The mainstays of debridement of acute wounds in veterinary medicine are surgical and mechanical debridement.
| Description | When | Example | |
|---|---|---|---|
| Primary closure | Debridement and immediately close wound | 24 hours | Minimal tissue damae or contamination and closed after initial debridement. Local tissue available for closure Delayed primary closure |
| Delayed primary closure | Debridement and open wound management prior to closure | 3–5 days | Mild to moderate tissue damage and contamination Closed after several debridement procedures |
| Secondary closure | Debride, period of open wound management and closure of wound once granulation tissue established | 5 days | Heavy contamination and severe tissue damage, wound managed until healthy granulation bed |
| Secondary intention | Debridement and open wound management until wound healed | Until healed | Tissue may not be available for closure, wound left to contract and epithelialise |
The following debridement techniques described will assume the wound has been suitably prepared as described in the first article (Aldridge, 2013).
Surgical (or ‘sharp’) debridement
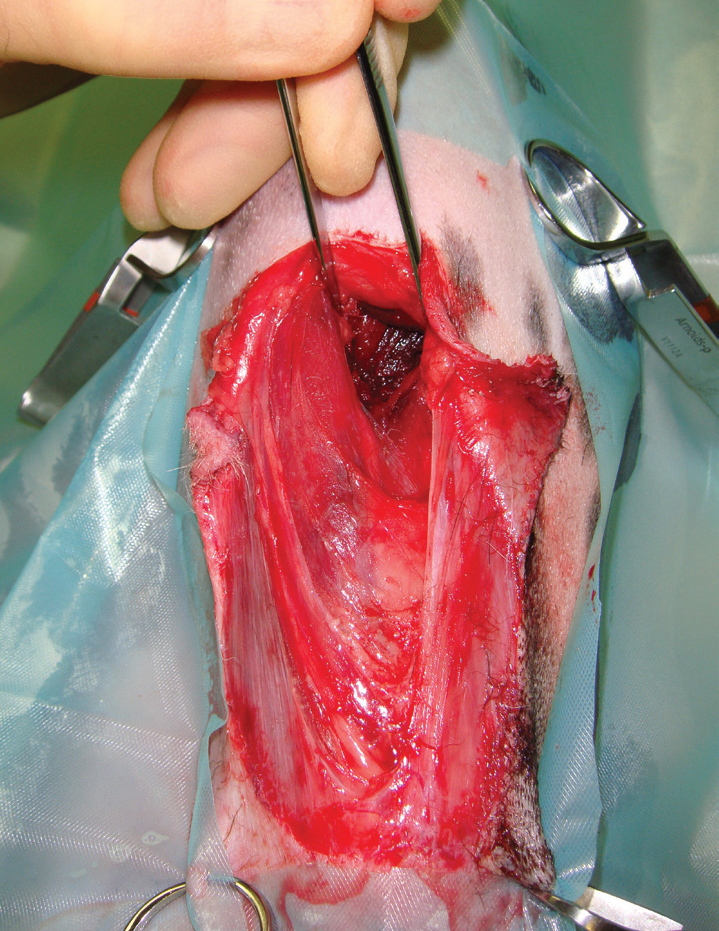
In surgical debridement, devitalised tissue is cut from the wound, allowing rapid and effective removal of tissue (Strohal et al, 2013) as well as thorough wound exploration (Dernell, 2006) and assessment of underlying vital structures (Figure 2). Debridement should be treated as any other surgical procedure; strict aseptic technique should be used to prevent introducing further bacterial contamination into the wound (Williams, 2009), therefore the area is draped, and the clinician gowned and gloved (Figure 3).
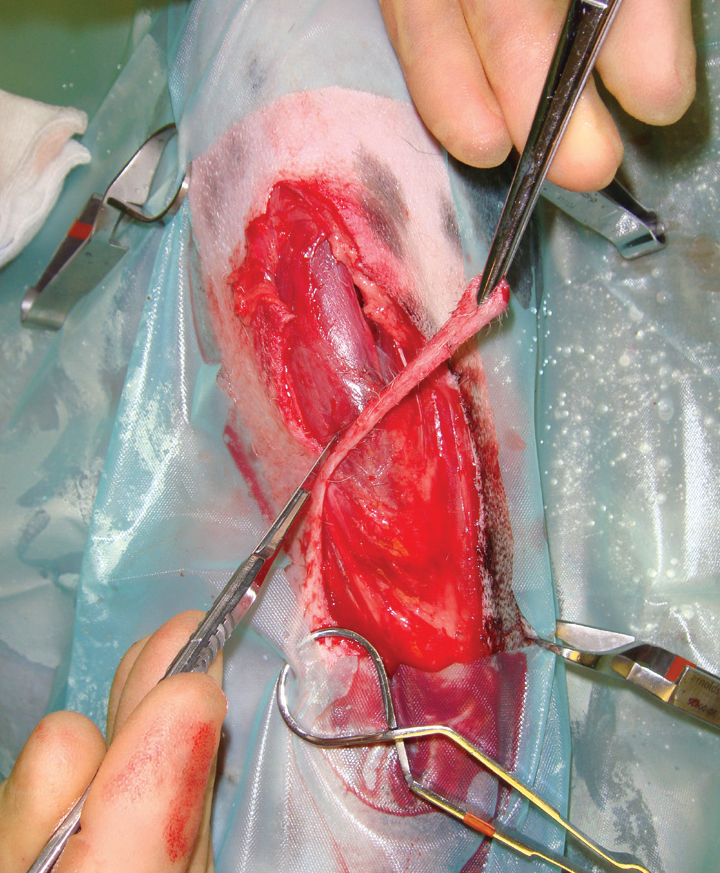
The aim is to remove devitalised tissue, while preserving the blood supply to healthy tissue; to achieve this the following points should be adhered to (Anderson, 1996):
Generally two approaches to surgical debridement can be used; ‘en bloc’ or layered debridement. The choice depends on the availability of surrounding tissue for closure, and therefore the need to preserve as much skin as possible.
En-bloc debridement
In areas where there is adequate normal tissue to allow closure afterwards, the whole affected tissue can be excised with a border of healthy tissue — in a similar fashion to removing a tumour with healthy margins in all planes. This is termed en-bloc debridement, ideal areas for this technique are the trunk, or proximal limbs where there is plenty of available skin to close the deficit. The wound is usually packed with surgical swabs and sutured closed prior to excision of the entire wound.
Layered debridement
More commonly when debriding a wound, devitalised tissue is removed gradually in layers, allowing conservation of tissue where possible (Anderson, 1996), this may be important in areas such as the lower limbs and feet where there is inadequate skin to allow en-bloc debridement.
Superficial tissue is removed first, followed by debridement of deeper tissues (Swaim and Henderson, 1997). As debridement progresses, instruments can be changed, or disinfected and rinsed to prevent contaminating areas that have already been debrided.
Assessing which tissue is non viable and should be removed is not as straightforward as it sounds. In areas of non-vital tissue (i.e. skin, muscle, fat), one option is to cut tissue back until it bleeds, on the understanding that haemorrhaging tissue is healthy tissue (Williams, 2009). Certain factors can affect the degree of bleeding from cut tissues (Dernell, 2006), such as systemic or local vasoconstriction, tissue temperature and coagulation defects, therefore this more aggressive approach could result in removing viable tissue.
Where removing viable tissue is an issue, due to the requirement to preserve as much tissue as possible, a more conservative approach is taken. A line of demarcation between dark (or very light) tissue and normal coloured tissue is a good indicator of non-viable tissue (Figure 4). Where there is doubt over an area it can be left and re-evaluated at the next assessment, when a line of demarcation may have developed, this is sometimes termed ‘staged’ debridement.
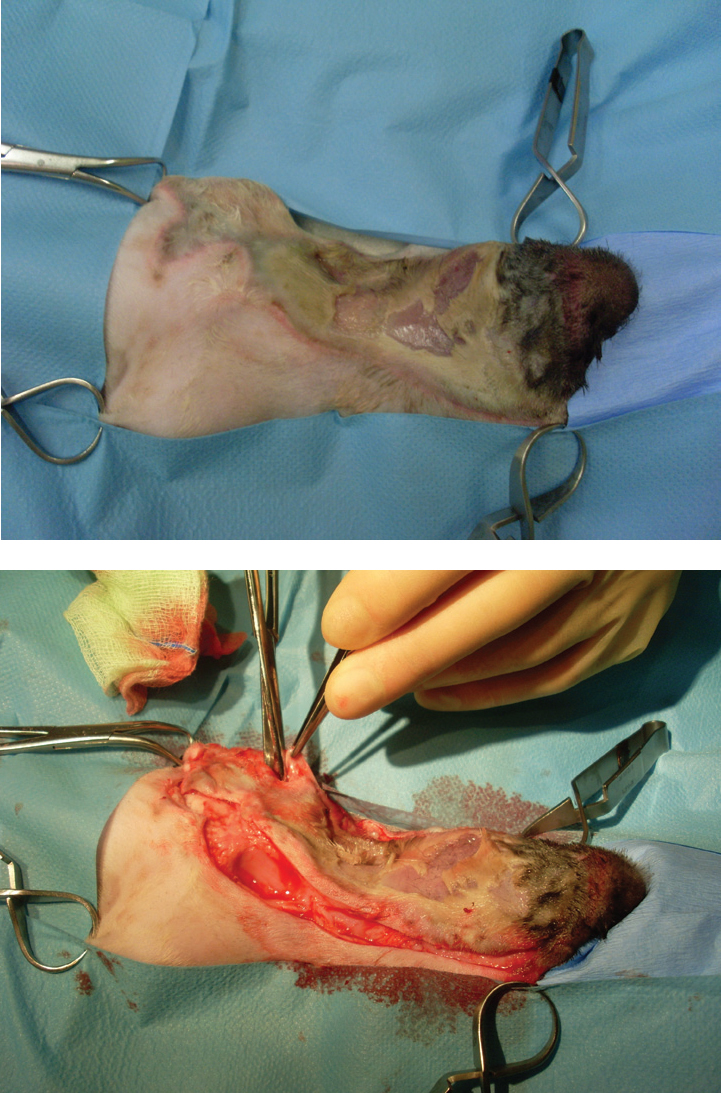
Copious lavage should be performed during surgical debridement, to flush debris and contaminants, and also to re-hydrate tissues, which assists in the assessment of tissue viability (see Aldridge (2013) for details of suitable solutions and techniques) (Figure 5).
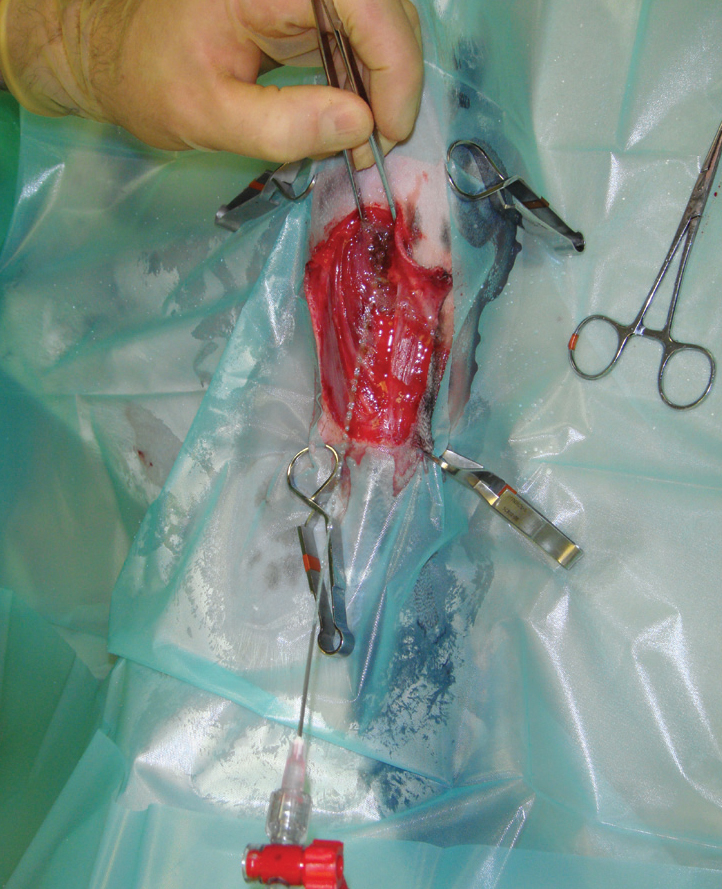
Surgical debridement is often followed by the use of dressings to achieve mechanical debridement. This is especially the case where a single surgical debridement is not sufficient to produce a wound bed suitable for closure. A common technique is to assess and surgically debride the wound daily, while using dressings in between procedures to mechanically debride the wound.
Mechanical debridement
Mechanical debridement utilises adherent dressings to remove non-viable tissue and foreign material from a wound bed (O'Dwyer, 2007).
Wet-to-dry dressings
Wet-to-dry dressings have been used for decades in humans (Armstrong and Price, 2004) and they are a very useful tool in wound management in veterinary practice (Aldridge and O'Dwyer, 2013). Their effectiveness relies on the adherence of the dressing to the wound bed (Figure 6), which on removal lifts the debris and necrotic tissue that becomes trapped in the mesh of the dressing.
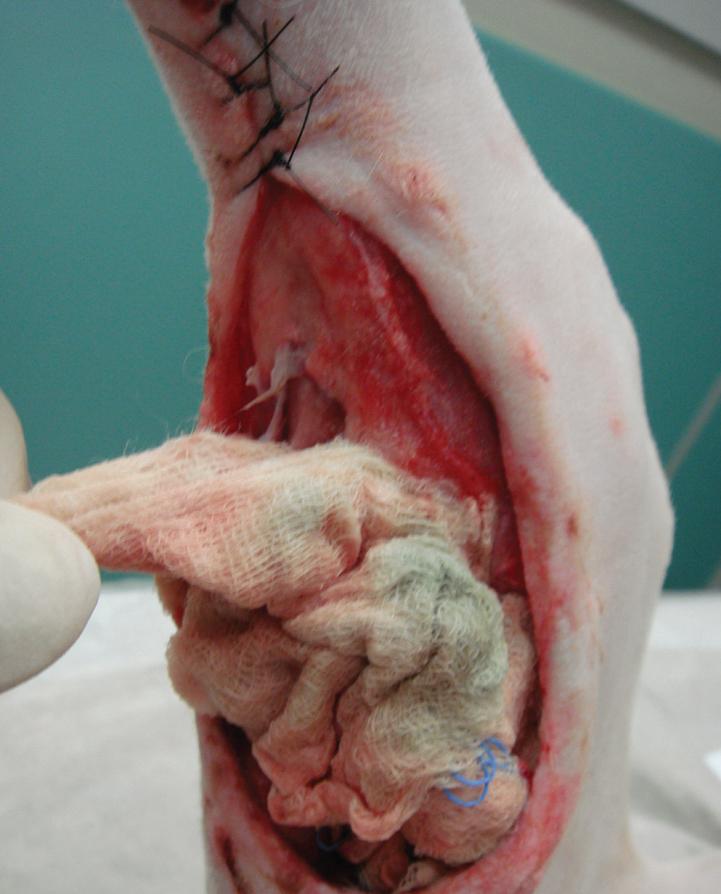
The application of wet-to-dry dressings is straightforward, and involves readily available materials (Table 2). A sterile gauze swab is wetted with sterile saline and placed in contact with the wound bed, this is then protected by standard secondary and tertiary bandage layers (O'Dwyer, 2007). The moisture from the swab dilutes the exudates in the wound, which is absorbed into the secondary layer (Figure 7), and the swab dries and adheres to the wound surface. The dressing needs to 1994). Removal is often painful, and sedation or anaesthesia is usually required
| Aseptic technique is used throughout. | |
| Sterile gauze swabs are wetted with sterile saline, and excess moisture squeezed out, to leave the swabs damp | |
| The swabs are packed into the wound bed, ensuring they are in contact with all of the wound surface; tucking into pockets and below skin margins | |
| Dry gauze swabs can then be placed over the damp swabs | |
| An absorbant secondary layer is placed and compressed with open weave bandage. Then a tertiary layer that allows evapouration is then placed over the primary layer | |
| The dressing is removed in 24 hours, or sooner if strike through is noted |
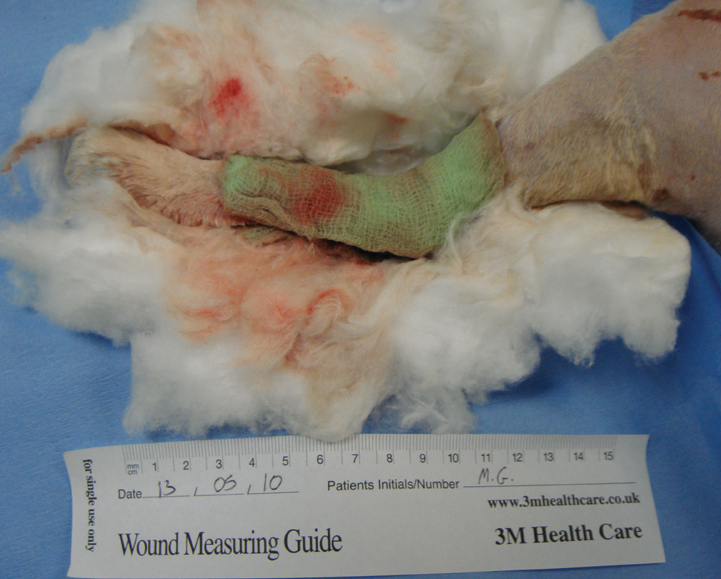
Wet-to-dry dressings are very useful, cost effective, and easy to apply; but if applied incorrectly can delay healing, common mistakes in technique that must be avoided are outlined in Table 3.
| Don't over wet the swabs. Wring them out well; otherwise they will not dry out and will not adhere to the wound | |
| Do make sure the wet swabs are in direct contact with a highly absorbent secondary layer | |
| Do place several layers of absorbent padding, sufficient is needed to absorb exudates before strike through | |
| Do make sure the wet swabs are in direct contact with the secondary layer |
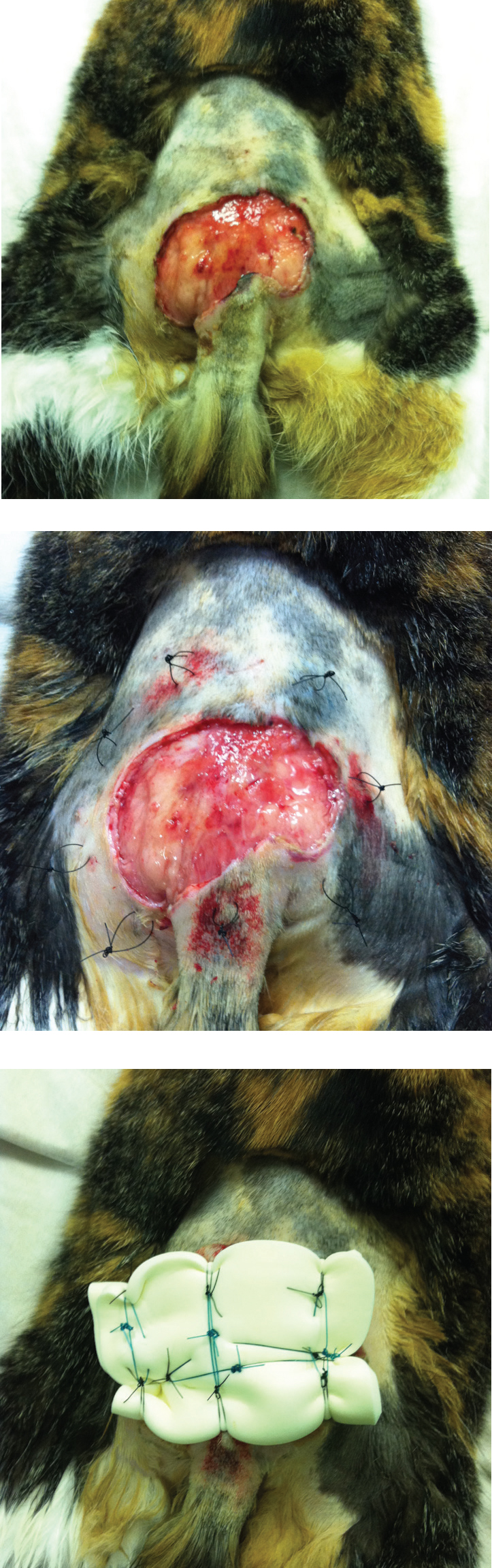
Debriding dressings can be used anywhere on the body; if they are used in areas that are difficult to bandage, such as the greater trochanter, or on the trunk, a ‘tie-over’ technique can be used (Williams, 2009; Dernell, 2006). In a tie-over dressing, loops of monofilament suture material are tied at intervals around the wound bed. The wet-to-dry dressing can then be placed over the wound bed, followed by cotton wool, or a laparotomy pad, followed by a tertiary layer. The dressing is then held in place by nylon tape threaded through the suture loops (Figure 8). During redresses the suture loops are left in place and utilised for the next dressing (O'Dwyer, 2007).
In wounds that are producing large amounts of low-viscosity exudates, a dry-to-dry dressing may be preferable (Anderson, 1996). The dressing is identical, other than the swabs are not wetted, and are placed onto the wound dry. This ensures that even though there is a large amount of exudate, the swab becomes wet, the fluid is absorbed into the secondary layer, and the swab becomes dry again.
Other debridement methods
Autolytic
Autolytic debridement is a selective debridement brought about by release of the patient's own proteolytic enzymes (e.g. collagenase, elastase) and the activation of phagocytes. These enzymes soften and breakdown necrotic tissue, and are mostly produced by leucocytes. This is a natural process that occurs in all wounds, but its action can be enhanced by using products that promote and maintain an ideal moist wound environment that promotes the activity of leucocytes and macrophages (Strohal et al, 2013). The moist environment also promotes the swelling of necrotic tissue, which loosens it from the wound bed.
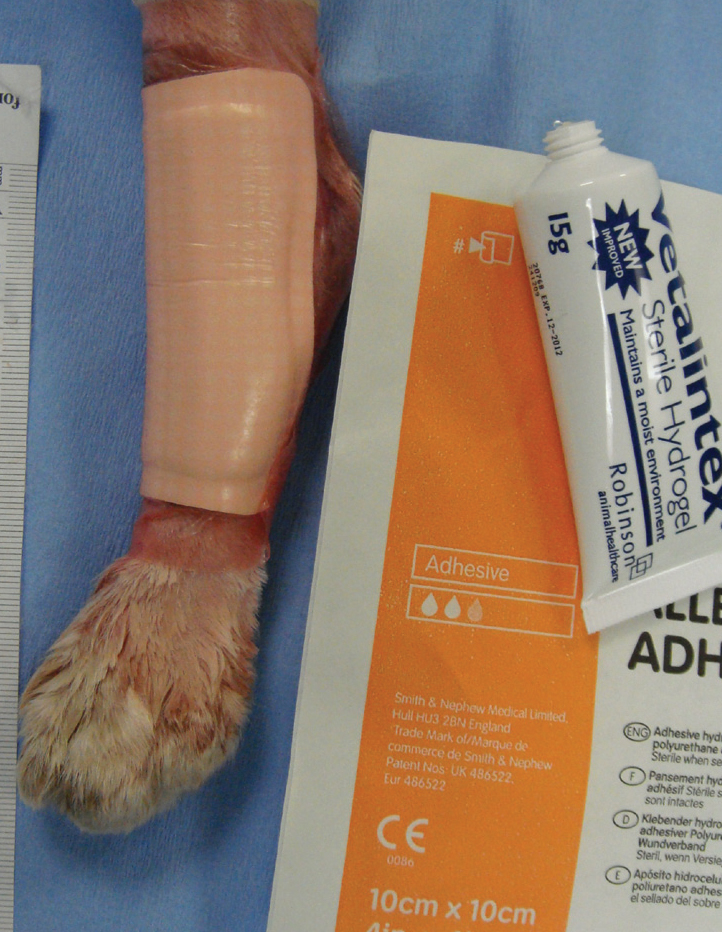
Hydrogels are polymers that are saturated with water, different gel forming agents such as carboxymethylcellulose are incorporated into most hydrogels (Dissemond, 2006). Hydrogels ‘donate’ water into the wound to promote a moist environment, they should only be used in wounds with moderate or no exudates (Figure 9). Hydrocolloids are composed of carboxymethylcellulose, gelatine, pectin, elastomers and adhesives that turn into a gel when exudate is absorbed, these absorptive dressings can be used in exudative wounds (Gray et al, 2011). Autolytic products do not damage healthy tissue, and are said to promote the formation of granulation tissue and epithelialisation.
Honey
Records of the use of honey extend back more than 4000 years (O'Dwyer, 2007). Honey is a supersaturated sugar solution containing 30% glucose, 40% fructose, 5% sucrose and 20% water as well as other substances such as amino acids, vitamins, minerals and enzymes. For wound management honey is available in tubes, or in impregnated dressings (Figure 10).
Honey promotes autolytic debridement, while having antimicrobial effectiveness. Honey osmotically draws fluid from the surrounding tissues, this reduces wound oedema and increases exudates which both promote autolytic debridement (Strohal, 2013). Antimicrobial activity is likely to be due to a number of factors; osmotic dehydration of bacteria, low pH (3–4.5), release of small amounts of hydrogen peroxide or methyglyoxal (Schneider et al, 2007).
Honey should not be used in dry wounds, where its osmotic effect would further dry out the wound (Strohal et al, 2013).
Enzymatic
Enzymatic agents are relatively selective in loosening and removing necrotic tissue. No veterinary product exists in the UK, but in the USA an ointment derived from bacteria (Bacillus subtillus), which contains the enzyme sutilain, is available and can be used in wounds with minimal necrotic tissue (Dernell, 2006).
A much wider range of proteolytic enzyme preparations are available for wound management in humans, including enzymes derived from plants (e.g. pineapple), bacteria, and animals (e.g. Antarctic krill (Mekkes et al, 1998)).
Larval
The use of maggots in wounds is an idea that has been around for hundreds of years (Sherman et al, 2000). In human medicine the most commonly used maggot is that of the greenbottle fly (Lucillia sericata). The advantage of maggots is that they selectively debride necrotic tissue while sparing healthy structures; this may be advantageous in areas where vital structures exist and aggressive surgical debridement is therefore contraindicated (Hendrix, 1991). In human use the maggots are restrained with a net incorporated into the dressing, and are removed after 3 days (O'Dwyer 2007).
Advances in debridement
Hydrosurgery
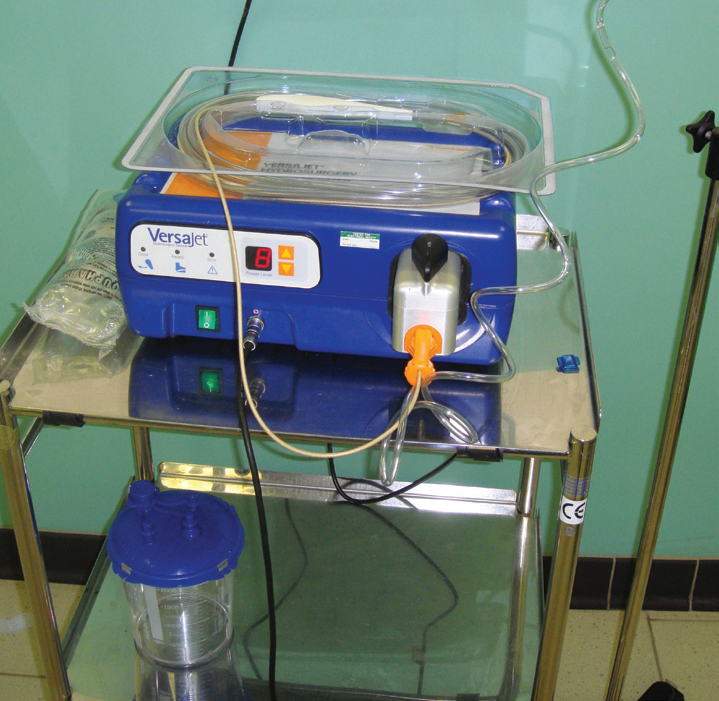
Hydrosurgical units emit a very high pressure stream of saline that cuts tissues. The Versajet (Smith and Nephew) is the most commonly used unit in human hospitals, where they are used for debridement of wounds, chronic ulcers, and burns (Rennekampff et al, 2006). These units should not be confused with ‘water-piks’ that deliver a stream perpendicular to the wound, and are used for pulsatile lavage. In a Versajet the stream crosses a window in the hand piece, and is orientated parallel to the wound bed, allowing tissue to be ‘shaved’ off; the saline passes into an evacuation collector (Figure 11) (Placek, 2007).
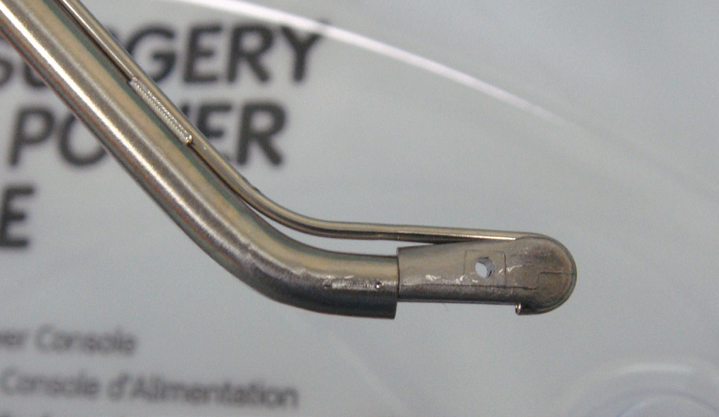
The Versajet not only cuts with the saline stream, but the stream creates a vaccum (‘Venturi’ effect) that evacuates the ablated tissue and debris along with the saline, leaving a clean wound bed. The speed of the stream is controlled on the console.
Human reports suggest that Versajet is equally, if not more effective than conventional surgical debridement, by causing less damage to viable tissue (Sainsbury, 2009). Other perceived advantages include shorter surgical time, shorter hospital stays, and less risk of injury or aerosol contamination compared with surgical debridement with lavage (Granick et al, 2006).
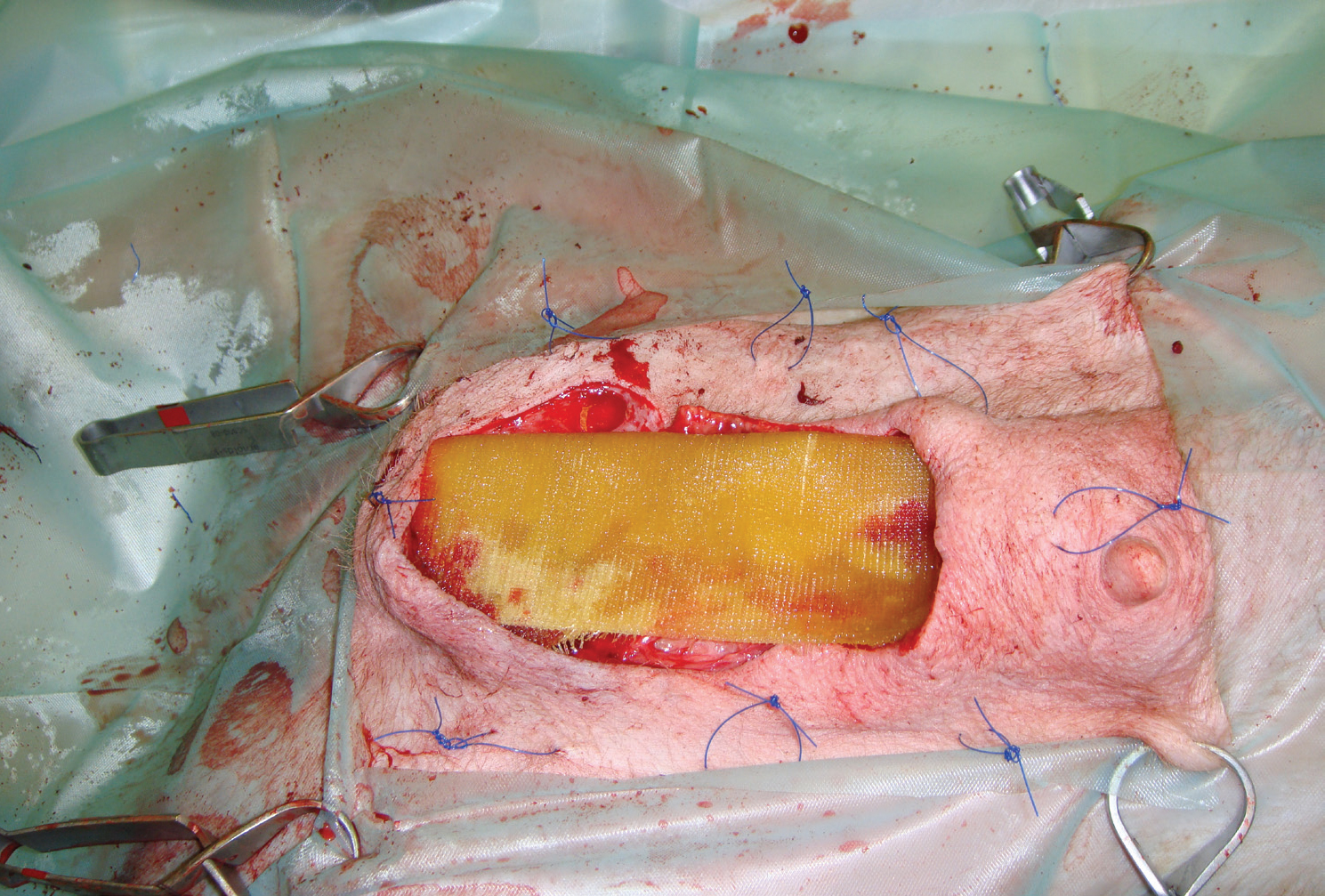
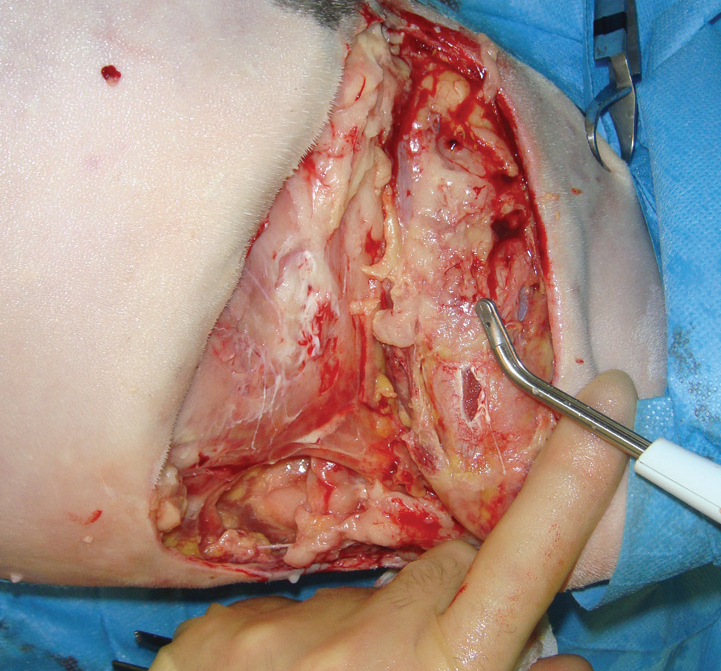
The author has a Versajet in his clinic, and finds it very useful in the layered debridement of wound where maintaining viable tissue is paramount, but a high level of contamperiosteum if required (Figure 12). It is also useful for the removal of subcutaneous tissue during the preparation of free skin grafts.
Conclusion
In some traumatic wounds it may be possible to debride the wound surgically in one procedure, e.g. a fresh laceration with minimal tissue damage, or by en-bloc debridement of a wound on the trunk. However, in more contaminated wounds, or in areas where the preservation of vital structures is imperative, or where maintaining as much viable skin as possible is important, often more than one procedure is required (a technique known as staged debridement). In staged debridement, surgical debridement can be repeated at intervals, with the use of additional debridement in between: mechanical, autolytic, or enzymatic. In the human field hydrosurgery is gaining in popularity due to the benefits in speed and establishing a clean wound bed.
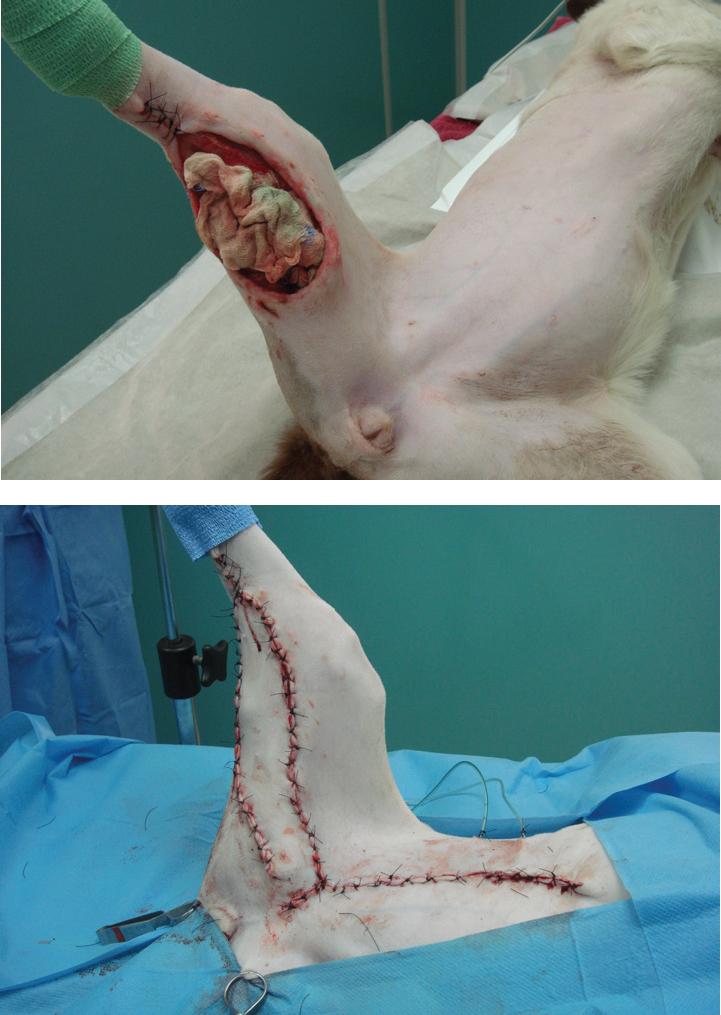
During the process of debridement, it is important to keep inspecting and re-evaluating the wound. As soon as the clinician is confidant that contamination and necrotic tissue has been reduced to a level where phagocytosis can deal with the remaining impediments to healing, wound closure can be considered. It is often more cost effective to reconstruct a wound at this point (Figure 13), rather than a prolonged period of second intention healing, which can rapidly run up a large bill with repeated dressings and sedations.