Hyperadrenocorticism (HAC), or Cushing's disease, is one of the most commonly diagnosed endocrinopathies in dogs (Herrtage and Ramsey, 2012), having been found in approximately 0.1% of the general canine population (Hill et al 2005). The condition results in excessive glucocorticoid production by the adrenal cortex of the adrenal glands. It is this chronically increased production of glucocorticoids that contributes to the negative effects on the patient's body. Because veterinary nurses play a vital roles in the diagnosis of HAC, it is important that they be able to identify the clinical signs of the disease, properly perform diagnostic tests, and understand the general treatment options available in order to achieve excellent client communication.
Normal physiology of the hypothalamus, pituitary gland and adrenal gland
The axis controlling glucocorticoid release is initiated by the release of corticotropin-releasing hormone (CRH) from the hypothalamus. The CRH in turn stimulates the anterior pituitary gland to produce adrenocorticotropic hormone (ACTH). The ACTH then travels systemically to the cortex of both adrenal glands to synthesise and secrete cortisol (a glucocorticoid). The cortisol levels in the blood negatively feedback on the hypothalamus and pituitary to decrease the synthesis and release of CRH and ACTH (Figure 1).
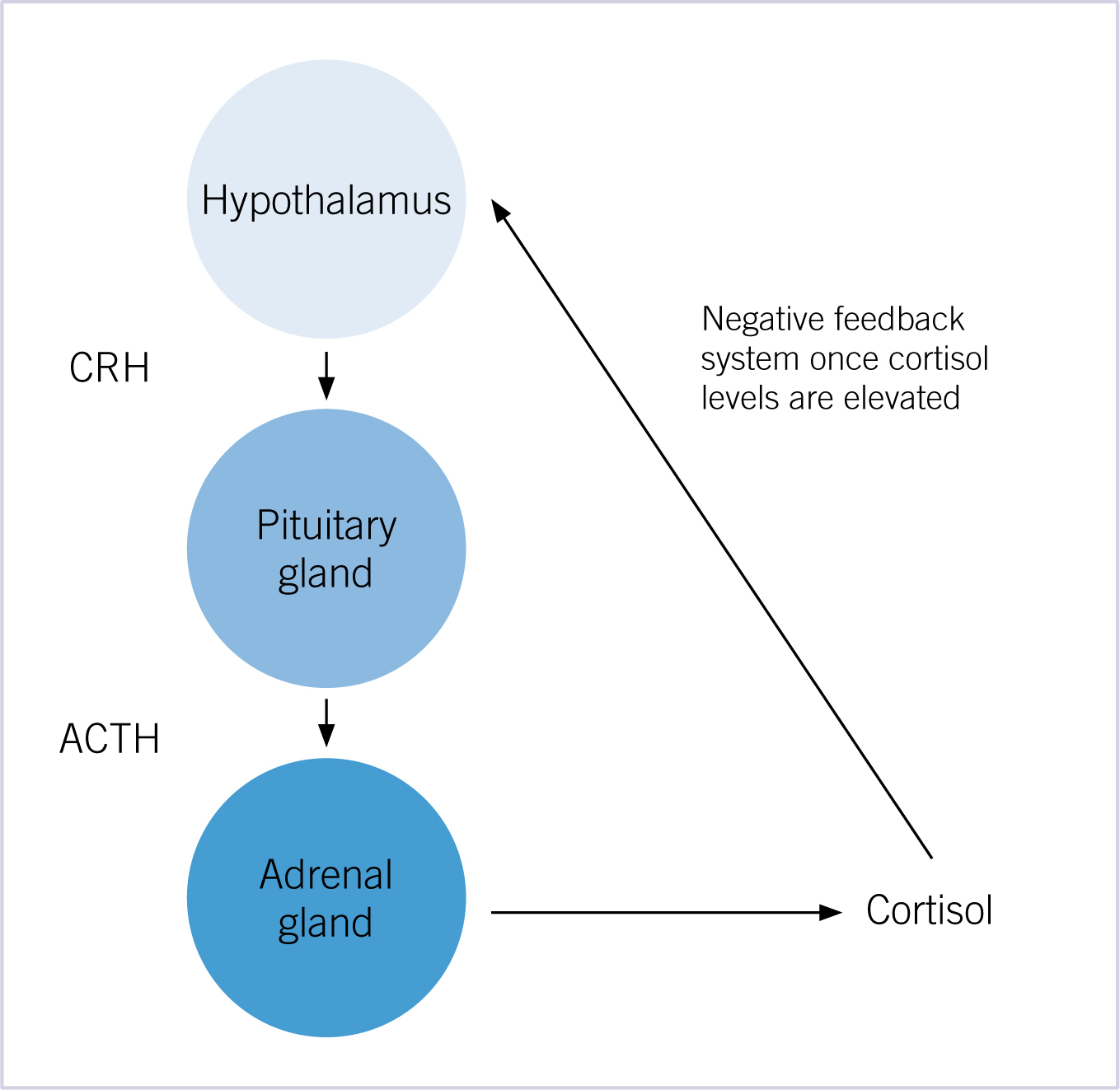
Pathophysiology of HAC
HAC can be pituitary-dependent, adrenal-dependent, or iatrogenic. There have been rare cases where dogs have had ectopic ACTH secretion from tumors located other than in the pituitary gland, or ectopic or eutopic hormone receptors that are ACTH-independent stimulated, all of which produce an excess amount of cortisol (Galac and Kooistra, 2012). There has also been awareness raised for ‘atypical HAC,’ which is where the patient has the classic history, physical examination, and certain clinicopathological findings, but the HAC diagnostic fails to identify elevated cortisol levels (Behrend et al, 2013). Discussion on diagnostic testing and treatment will be focused on pituitary-dependent and adrenal-dependent HAC.
Pituitary-dependent HAC (PDH)
The pituitary form of HAC accounts for 80–85% of HAC cases (Reusch, 2005; Galac and Kooistra, 2012). In PDH, excess ACTH secretion is caused by either pituitary hyperplasia, pituitary adenoma, or less commonly, a pituitary carcinoma (Reusch, 2005). It is estimated that as high as 90% of dogs with PDH have a pituitary tumor (Herrtage and Ramsey, 2012). In PDH, the stimulatory effect of excess ACTH secretion causes hyperplasia of both adrenal cortices and results in excess cortisol release.
Adrenal-dependent HAC (ADH)
The adrenal form of HAC accounts for 15–20% of HAC cases (Reusch, 2005; Galac and Kooistra, 2012). In contrast to PDH, an adrenal tumor autonomously secretes cortisol ignoring the normal axis signal mediated by ACTH. The increased cortisol levels stimulate the negative feedback system resulting in suppression of ACTH production, reduced stimulation of the non-tumor bearing adrenal gland, and subsequent atrophy of its adrenal cortical tissue (Reusch, 2005). Adrenal tumors can be either benign or malignant (Reusch, 2005). Dogs with ADH usually only have one adrenal tumor, but bilateral tumors have been found in about 10% of cases (Galac and Kooistra, 2012). It is hard to determine whether an adrenal tumor is benign or malignant based on non-invasive methods. Certain criteria have been reported to suggest that an adrenal mass is malignant. These have included mass size larger than 4 cm, and evidence of vascular or lymphatic invasion via ultrasonography (Reusch, 2005).
Signalment
HAC usually affects middle aged to older dogs (Reusch, 2005). There is no sex predisposition, but certain breeds appear to be more predisposed than others: Poodles, Dachshunds, Beagles, various terrier breeds, and Boxers (Reusch, 2005).
Clinical signs
There are a number of clinical signs, some or all of which, will be present in patients with HAC (see Box 1 for a condensed version of clinical signs):
Polyuria/polydipsia
Polyuria and polydipsia are among the most common clinical signs noted by owners. There could be many potential causes of polyuria, including: cortisol causing an increase in glomerular filtration rate; cortisol inhibiting the release of the antidiuretic hormone (ADH) from the pituitary gland; and cortisol causing inhibition of ADH action on the renal tubules. Polydipsia then follows polyuria (Herrtage and Ramsey, 2012).
Polyphagia
The exact direct mechanism by which increased cortisol levels causes polyphagia is still unknown, but one theory is that glucocorticoids may have a direct effect on the appetite centre in the central nervous system (Herrtage and Ramsey, 2012).
Panting
The causes of panting could be multifactorial. Glucocorticoids enhance muscle wasting, which could include the skeletal muscle that surrounds the thoracic cavity, which would not allow the patient to expand its lungs to the full extent. Another cause could include hepatomegaly (commonly seen in Cushinoid patients), which would push the diaphragm up, not allowing the patient to expand its lungs and chest to their full extent.
Abdominal distention
This is due to liver enlargement, abdominal muscle wasting with resulting poor abdominal muscle tone, and redistribution of fat to the abdomen, giving the patient a ‘potbelly’ look (Figure 2).
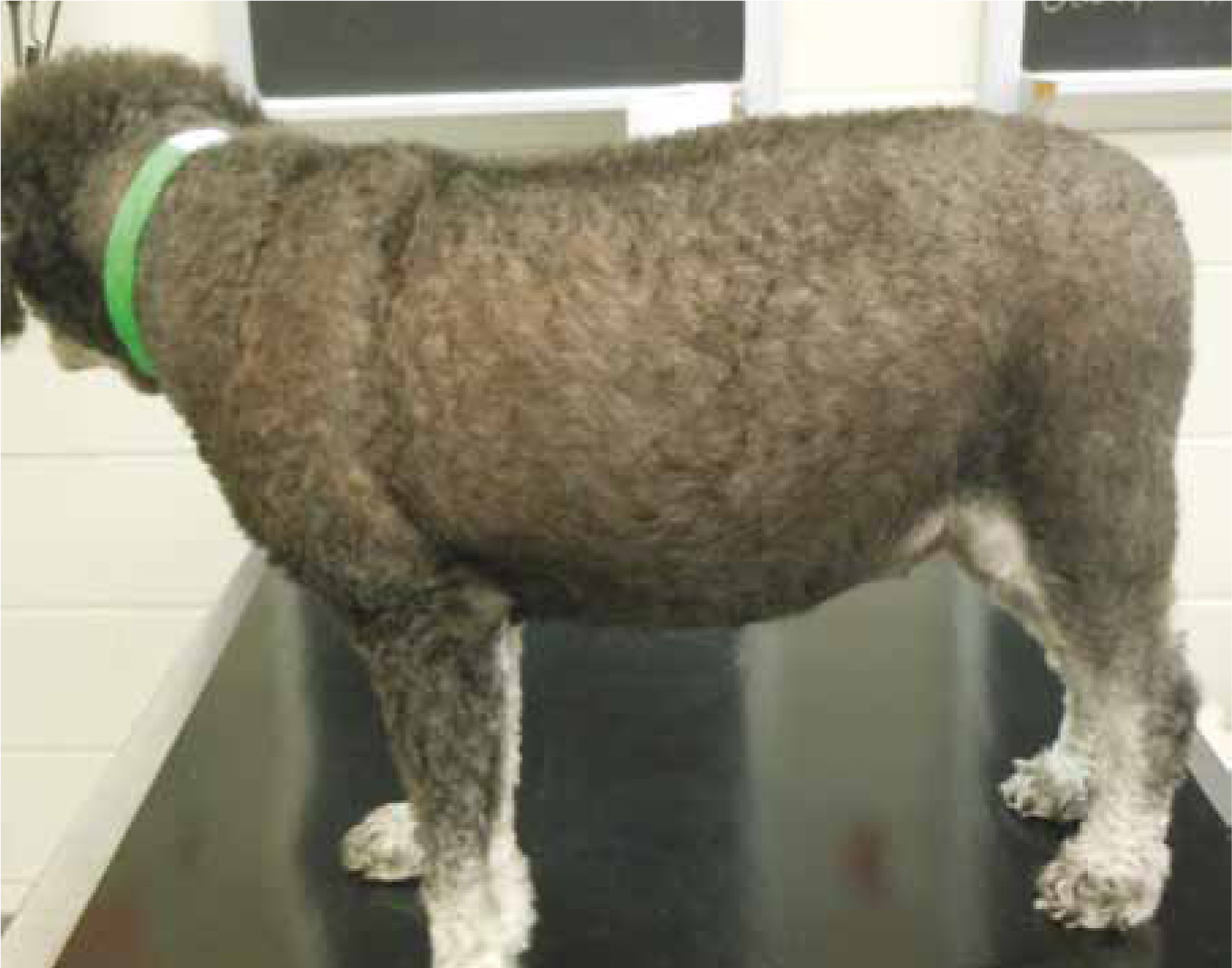
Muscle wasting
Glucocorticoids (cortisol) enhance catabolism of proteins in the body in order to provide amino acids for gluconeogenesis. This catabolism can lead to muscle wasting (Bill, 2006). Muscle wasting can lead to exercise intolerance. Because the muscles surrounding the abdomen atrophy from catabolism, abdominal organs can be more easily palpated.
Dermatological abnormalities
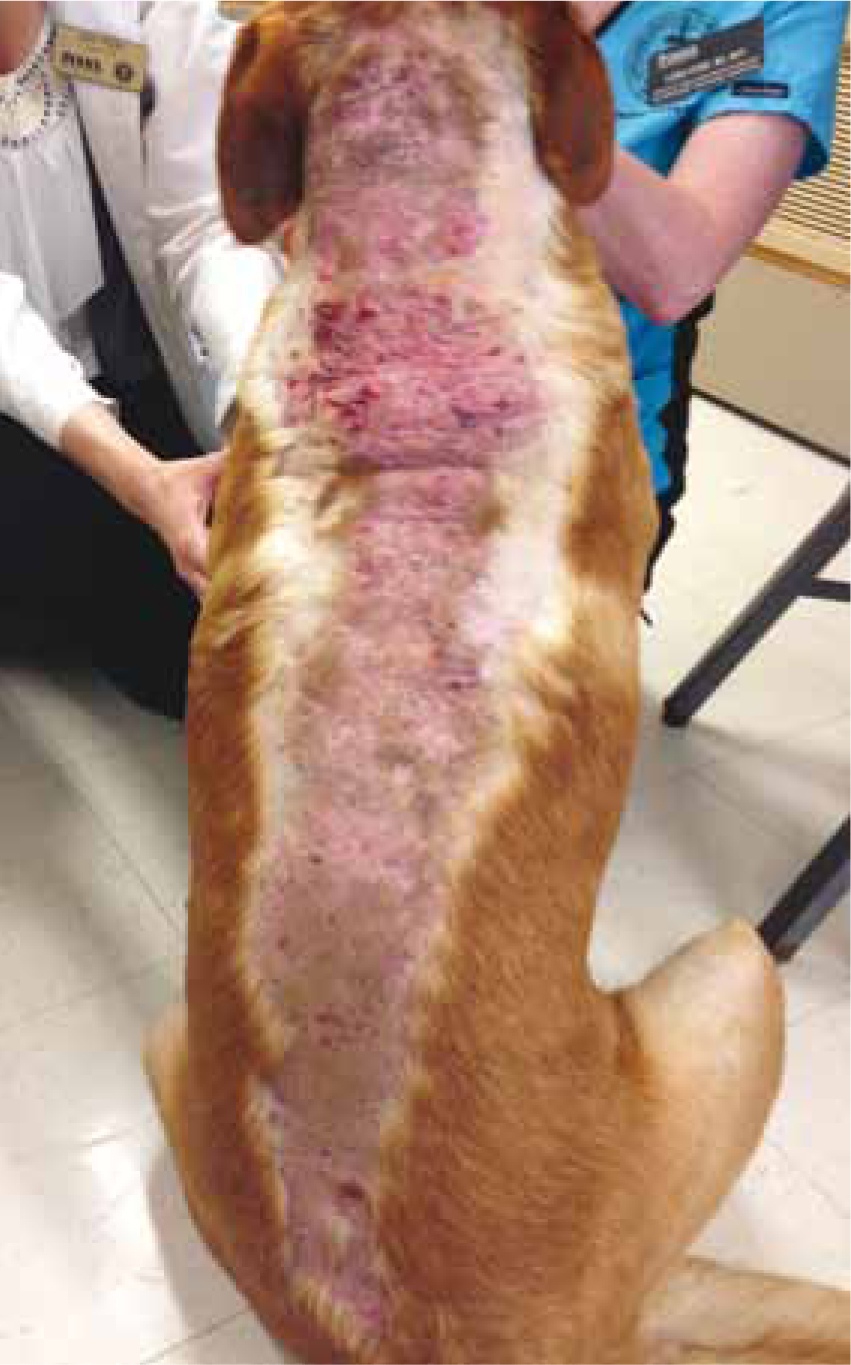
Dermatological signs include bilateral alopecia on the flanks, ventral abdomen, chest, perineum, and neck (Figure 3). When clipped, hair may fail to grow back (Herrtage and Ramsey, 2012). Wound healing may be slow due to glucocorticoid's suppression of fibroblast deposition of fibrin and wound closure (Bill, 2006). Calcinosis cutis is calcification of the dermis and subcutis frequently observed in histopathology findings from biopsies (Herrtage and Ramsey, 2012). It is most commonly found on the neck, inguinal areas, ventral abdomen, and axillae (Herrtage and Ramsey, 2012) (Figures 4 and 5).
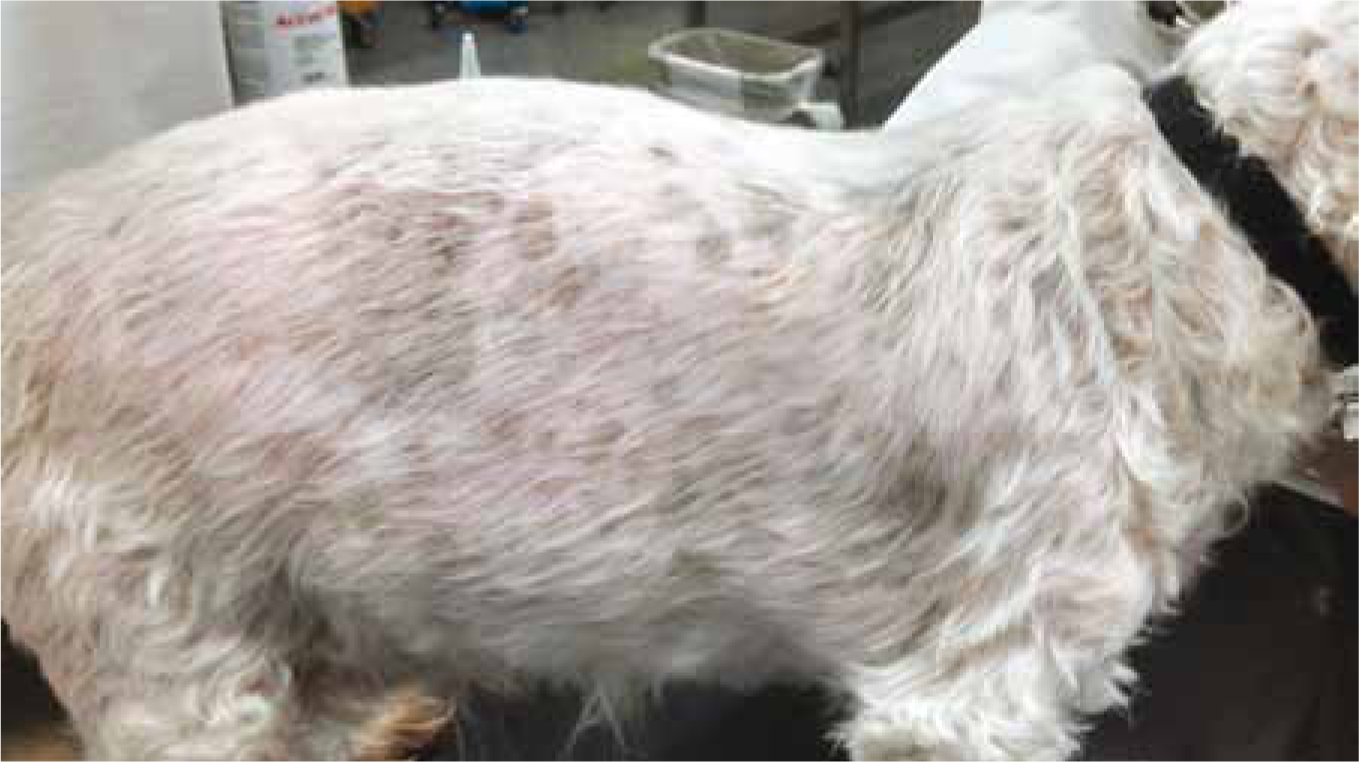
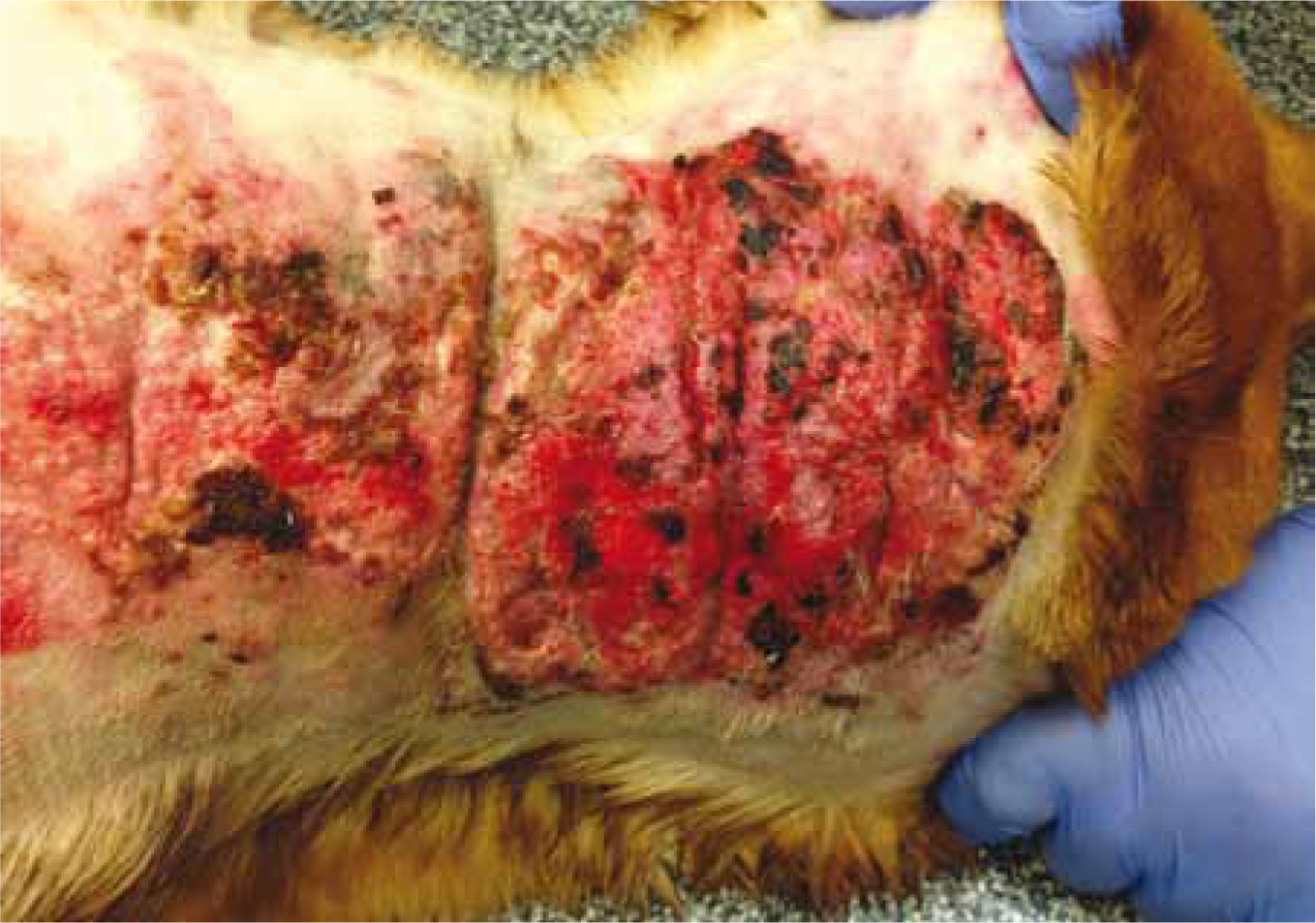
Reproductive abnormalities
Testicular atrophy and cessation of oestrous cycling are due to decreased pituitary gonadotropic hormones (Reusch, 2005). Due to the high concentrations of cortisol, the negative feedback system suppresses ACTH as well as the other gonadotropic hormones from the pituitary gland (Herrtage and Ramsey, 2012).
Neurological abnormalities
Dogs with large pituitary tumors may present with central nervous system (CNS) signs, depending on the extent to which the tumor impinges on adjacent neural tissue (Herrtage and Ramsey, 2012). Common clinical signs would include dullness, depression, anorexia, wandering/pacing, ataxia, headpressing, disorientation, circling, aniscoria, and seizures (Herrtage and Ramsey, 2012).
Hypertension
Hypertension occurs in >50% of dogs with untreated HAC (Herrtage and Ramsey, 2012). There are different ideas as to why hypertension is associated with Cushinoid patients:
Pulmonary thromboembolism
Pulmonary thromboembolism is a rare but potential concern for patients that have HAC. Signs of pulmonary thromboembolism would be hyperpnea to dyspnea due to obstruction of pulmonary arterioles (Herrtage and Ramsey, 2012).
Myopathy
In some cases of HAC, dogs develop a myotonia, where their muscles do not relax (Herrtage and Ramsey, 2012). Normally seen in the hindlimbs, it can be noticed as a stiff gait, but can also progress to muscle hypertrophy from constant stimulation to contract (Herrtage and Ramsey, 2012).
Clinicopathological findings
There are a number of clinicopathological finds associated with HAC:
Diagnostic imaging findings
Additional diagnostics include the following:
Hormone tests to screen for HAC
Urine cortisol:creatinine ratio is used as a screening test and is not specific for HAC as the sensitivity of this test is high (75–100%), but the specificity is low (20–25%) (Behrend et al, 2013). A patient that has an elevated urine cortisol:creatinine ratio can have many different differentials aside from HAC, and therefore requires subsequent testing for confirmation. However, a dog that has a normal urine cortisol: creatinine ratio is unlikely to have HAC.
It is better to collect a morning urine sample over several days, preferably by the owner at home.
Hormone tests to diagnose and classify ADH versus PDH
ACTH stimulation test
The ACTH stimulation test has a lower specificity (Behrend et al, 2013) for diagnosing HAC, but it is the only test that can determine iatrogenic cause of HAC. It does not distinguish between ADH or PDH (Reusch, 2005). This test is also performed on patients already diagnosed with HAC to monitor their response to treatment.
The test is best done in the morning following a 12 hour fast. Stress should be minimised as much as possible to reduce the chance of falsely elevating cortisol. Types of samples will depend on the laboratory used, but at Purdue University Veterinary Teaching Hospital (PUVTH), a pre sample of serum will be taken to identify a baseline cortisol concentration, then synthetic ACTH will be administered intravenously (IV) preferably or intramuscularly (IM) (5 µg/kg). One hour later, a serum sample will be taken for determining cortisol levels.
If the animal has iatrogenic HAC, the presence of administered exogenous corticosteroids will have caused negative feedback on the pituitary gland, decreased levels of ACTH, reduced stimulation of the adrenal glands, and subsequent atrophy of the adrenal cortex. When the ACTH is given by injection, the atrophied adrenal glands will not be able to respond to the ACTH stimulation and the concentrations of cortisol will not increase a normal amount. If the HAC is not iatrogenic, hyperplastic adrenal glands will produce large amounts of cortisol when stimulated by exogenous ACTH. Further tests need to be performed to determine whether the HAC is ADH or PDH.
Low-dose dexamethasone test (LDDST)
The LDDST test is a more sensitive test (85–100%) than the ACTH stimulation test (57–95%) in determining whether the patient has HAC or not (Behrend et al, 2013). It will not determine if the cause is iatrogenic, but it may distinguish between PDH and ADH.
This test is an 8 hour test and is best performed in a stable patient in a low stress environment so that it does not falsely elevate cortisol levels. Types of samples will depend on the laboratory used, but at PUVTH, a pre-serum sample is drawn to determine the baseline cortisol concentration followed by a dose of dexamethasone sodium phosphate (SP) or dexamethasone in polyethylene glycol given IV (0.01 mg/kg). If dexamethasone SP is used, the dose should be based on the concentration of dexamethasone in this drug (Behrend et al, 2013). A 4-hour post-injection serum sample is drawn followed by a 8-hour post-injection serum sample for cortisol analysis.
This dose of steroid should suppress the pituitary release of ACTH by negative feedback and subsequently decrease adrenal gland stimulation for 24–48 hours (Reusch, 2005). In a dog that has HAC, the cortisol levels will remain increased after 8 hours. This is why the LDDST is a good diagnostic test for HAC. If HAC is confirmed with the LDDST, the 4- or 8-hour post-injection serum cortisol concentration can potentially indicate if the patient has PDH. If it does not, further diagnostics need to be performed in order to confirm the patient has ADH versus PDH.
Endogenous ACTH
The test for endogenous ACTH measures the amount of ACTH present in the patient's blood and is used to help distinguish between ADH and PDH.
Samples for this test need to be collected in plastic EDTA tubes, rapidly separated, and sent to the laboratory frozen on ice to minimise the rapid breakdown of the ACTH giving falsely low results (Gilor and Graves, 2011).
If the ACTH levels are elevated, then the pituitary gland is producing excessive ACTH and the patient is likely to have PDH. Because ACTH is released episodically and not continuously from the pituitary gland, concentrations of ACTH may be low in the blood even though a pituitary tumor is present (Herrtage and Ramsey, 2012).
Treatment of PDH
Mitotane
Mitotane is an oral adrenocorticolytic drug that destroys the zona fasciculata and zona reticularis of the adrenal glands (Herrtage and Ramsey, 2012). As a result production of cortisol decreases in spite of the continued production of excessive ACTH. Because the owner is going to monitor the effectiveness of this drug at home, excellent client communication is required to use this drug properly. For initial treatment, the owner identifies a clinical sign(s) they feel they can easily monitor, such as amount of water or food intake. Owners are then dispensed the initial mitotane treatment to administer to the patient for up to 7–14 days (Herrtage and Ramsey, 2012). They are also supplied with a small amount of glucocorticoid drugs in case the cortisol levels drop so low they precipitate into an addisonian crisis (anorexia, vomiting, diarrhoea, and lethargy may be seen; more severe clinical signs could include collapse and dehydration along with electrolyte imbalances). When owners start to notice signs of decreased thirst (<60 ml/kg/day), or decreased appetite, or if they notice vomiting, depression, or diarrhoea, they are instructed to bring the patient to be re-evaluated and for an ACTH stimulation test in order to make sure the cortisol values to do not go too low and the patient then becomes addisonian. Veterinary surgeons and veterinary nurses may choose to call owners during this initial treatment several times to monitor the patient's progress. The patient is brought in at the end of the initial mitotane treatment, or when they feel any of the clinical signs noted previously have occurred for an ACTH stimulation test. If the treatment has been successful, the stimulation test will be suppressed (Herrtage and Ramsey, 2012).
Once both the pre- and post-cortisol levels are suppressed in spite of ACTH stimulation, maintenance treatment is initiated to prevent the adrenal cortex from regenerating under the continued stimulation by the ACTH from the pituitary tumor (Herrtage and Ramsey, 2012). One month after starting maintenance therapy, patients are checked using an ACTH stimulation test, and then rechecked thereafter, every 3–6 months (Reine 2012). Medications will be adjusted according to the tests as well as clinical signs.
Trilostane
Trilostane is an oral drug that inhibits the production of corticosteroids made by the adrenal glands by inhibiting the 3β-hydroxysteroid dehydrogenase enzyme system (Herrtage and Ramsey, 2012).
Once this drug has been started, ACTH stimulation tests should be performed to monitor cortisol levels and determine if the dose should be altered. Alterations should be small, and if the dose of trilostane is being increased, it should not be increased more than 50% of the current dose (Herrtage and Ramsey, 2012). ACTH stimulation tests should be performed after 10 days, 4 weeks, and 3 months of beginning trilostane (Herrtage and Ramsey, 2012). Once stabilised, ACTH stimulation tests only need to be performed every 3 months and after making any dose adjustments (Herrtage and Ramsey, 2012). It is important to remember that when performing ACTH stimulation tests on patients that are taking trilostane, the tests must be performed 4–6 hours post pill administration because the effects of trilostane are very short acting (Herrtage and Ramsey, 2012). This drug is usually well tolerated in dogs, but some adverse effects could include vomiting, diarrhoea, hyperkalaemia, hypoadrenocorticism (Addison's disease), and rarely, acute adrenal necrosis which may be fatal (Herrtage and Ramsey, 2012).
Surgical procedures
Bilateral adrenalectomy could be performed but is not commonly done in dogs unless they are resistant to other forms of therapy (Herrtage and Ramsey, 2012). Hypophysectomy (removal of the pituitary gland) is rarely performed in the United States.
Treatment of ADH
Surgical procedure
Surgical resection of the adrenal tumor is the ideal choice of treatment for ADH patients. Post-surgery patients require special monitoring, as they will become addisonian temporarily after the procedure due to the normal adrenal tissue being atrophied. Glucocorticoid supplementation will always be necessary and mineralocorticoid supplementation may sometimes be necessary based on measurements of electrolytes (Herrtage and Ramsey, 2012).
Mitotane
Dogs with ADH usually need a higher induction dose of mitotane than dogs with PDH, as well as a higher maintenance dose (Herrtage and Ramsey, 2012) due to adrenal tumors being resistant to this cytotoxic drug (Reusch, 2005). ADH dogs on mitotane need to be monitored closely with ACTH stimulation tests. Since these patients are on higher doses of mitotane, they are more at risk of developing adverse reactions (Herrtage and Ramsey, 2012). The tumor still has the potential to grow, despite the effects of mitotane.
Trilostane
Trilostane can also be used in ADH canine patients, but it may only help with the clinical signs ADH causes (Herrtage and Ramsey, 2012). It will not treat the tumor since its mechanism of action is blocking the synthesis of corticosteroids and not controlling the neoplastic cells of the tumor (Herrtage and Ramsey, 2012).
Conclusion
Understanding the disease, diagnostics, and treatment of HAC is important for the veterinary nurse. Veterinary nurses play a key role in obtaining histories and observing for clinical signs as they are often the first ones to see clients and patients. Some of the diagnostic endocrine tests have specific protocols to be followed, and often veterinary nurses will be the ones performing them, making it imperative to understand what these tests evaluate and how to properly perform them. The drug and surgery treatment plans involve a keen understanding of what these plans will and can do to the canine patient. Veterinary nurses need to understand these options in order to care for the patient and have excellent client communication.

