The clinical management of otic infection with Pseudomonas spp. is challenging. The disease is painful and debilitating and the pathogen is commonly a multiple-resistant isolate. Infection often progresses to involve the middle ear, which makes selection of medication a difficult balance between the clinical benefits of the use of off-license drugs versus the potential damage caused by the use of ototoxic medication. This article on the management of Pseudomonas otitis has been written as a series of frequently asked questions, designed to help nurses understand and help in the management of this difficult disease.
What is Pseudomonas spp.?
Pseudomonas species are Gram-negative, rod-shaped bacteria. They are widespread in nature but are particularly found in aquatic habitats, soil and in decaying vegetation. Pseudomonas aeruginosa is the species most commonly implicated as a cause of otitis externa/media in the dog. It is recognised as an opportunistic pathogen (Paterson, 2012).
Is Pseudomonas otitis a primary cause of otitis externa?
Pseudomonas is not a primary cause of otitis, but an infection that develops secondarily to the primary cause (Paterson and Matyskiewicz, 2018). Primary causes such as allergy, ectoparasites and endocrine disease are the inciting factors that lead to the development of otitis in both cats and dogs (Paterson and Matyskiewicz, 2018). They need to be addressed in all cases of otitis but especially in chronic disease where there is an increased risk of the development of multiple-resistant pathogens such as P. aeruginosa. Management of the underlying primary cause is essential to speed the resolution of the infection and prevent recurrence of infection, which is important to reduce antibiotic usage and reduce the risk of the development of more resistant forms of bacteria.
What does the discharge look like in cases of Pseudomonas otitis?
Infection with Pseudomonas typically produces a haem-orrhagic green/yellow, mucoid malodorous discharge, although on occasions it can present with a dark brown mucoid discharge (Figure 1). Although the clinical appearance of the discharge is very characteristic in cases of Pseudomonas infection, it should not replace cytological examination and subsequent culture and susceptibility when rods are identified (Barnard and Foster, 2018).
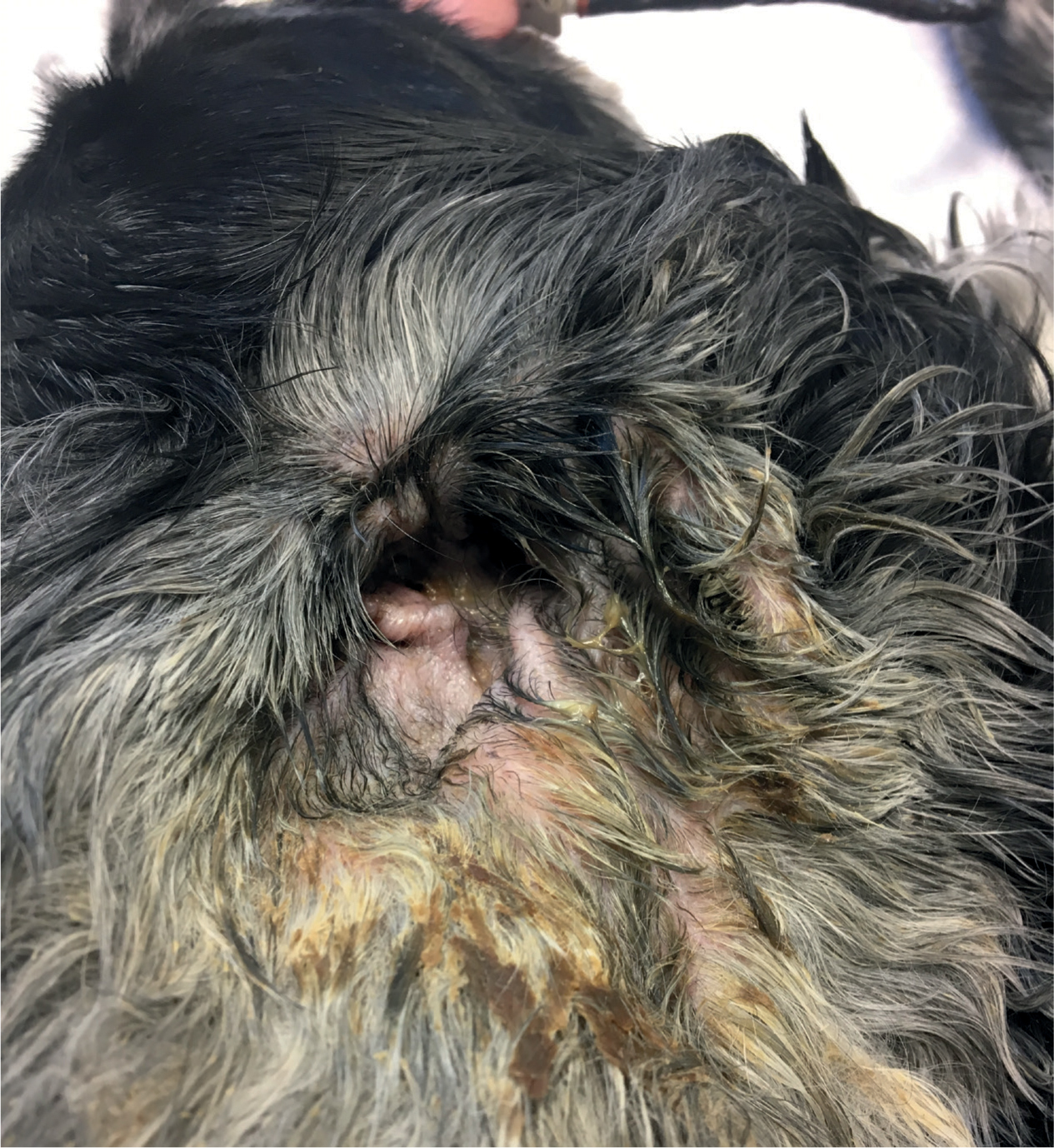
What does the ear canal look like in cases of Pseudomonas spp. otitis?
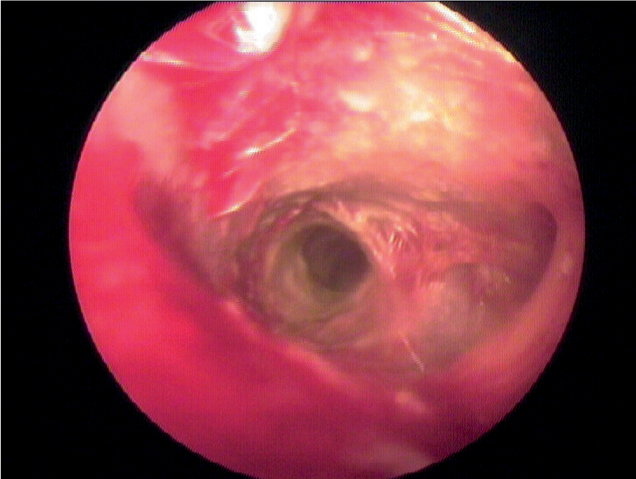
Pseudomonas spp. produces an array of exotoxins that causes severe damage within the ear canal (Lundman et al, 1992). Typically toxin elaboration produces a canal that is swollen, ulcerated and painful (Figure 2). It is therefore important when the dog is handled for examination, sample collection or when medication is applied to the ear that this should be undertaken in a sympathetic way to avoid unnecessary pain or apprehension on the part of the patient. In many cases dogs may need sedation or anaesthesia in order to be examined effectively and the author would suggest that pain relief should be prescribed as part of the treatment protocol in all cases.
How do I take a cytological sample from the dog's ear when I am suspicious there is Pseudomonas infection present?
Samples of discharge can be taken from the pet's ear with a cotton tipped swab. The sample is best collected from the junction of the horizontal and vertical canal by rotating a swab along the wall of the canal before rolling it onto a microscope slide. Sample collection should be performed carefully as the ear is usually ulcerated and painful. The ear swab may then be rolled along the microscope slide to transfer the mucopurulent exudate to the slide. The sample should be heat fixed, with a hand drier or hair drier, and then stained with a modified rapid Romanowsky stain such as DiffQuik or Rapi-Diff. It should then be examined. It is important when an otic sample is taken that sufficient material is left on the swab after the cytology sample has been made, for submission for culture and susceptibility if needed, otherwise a second sample should be taken at the time of the initial examination.
What does Pseudomonas spp. look like on cytology?
Cytological examination of the discharge is essential in helping to decide if Pseudomonas spp. is a likely cause of the infection. While it is good practice to undertake the initial examination of any cytological sample on low power, it is difficult to assess the morphology of Pseudomonas spp. unless oil immersion is used. A low power scan of the sample first allows the clinician to assess whether there are any parasites such as Demodex canis present and also facilitates selection of a stained area of the sample for subsequent viewing under a high power oil immersion objective. A Romanowsky stain such as DiffQuik or Rapi-Diff allows the identification of rod shaped organisms that are typical of Pseudomonas spp. together usually with a degenerate neutrophilic infiltrate. Although a Romanowsky stain provides good detail of the morphology of organisms on cytology samples it will not allow accurate differentiation between Pseudomonas spp. and other Gram-negative rods such as Proteus spp. or Escherichia coli, or Gram-positive rods such as Corynebacterium spp. Therefore where rods are identified on cytology, culture and susceptibility testing should be performed.
Why is culture and susceptibility important when cytology is suggestive of Pseudomonas otitis?
Where cocci or yeast are identified on cytology empirical therapy with a suitable drug is an appropriate first step in the management of the otitis. However where rods are identified, the susceptibility pattern of the organism is less predictable and culture and susceptibility are important to allow the selection of appropriate medication. While this additional expense can be difficult to justify to an owner, it should be communicated to them that it allows the accurate selection of an appropriate topical drug on the first occasion, helping to resolve the infection more quickly and reduce the risk of the development of a resistant pathogen.
Pseudomonas spp. is said to be capable of forming biofilms in cases of otitis — what is a biofilm and why is it important?
Micro-organisms are known to exist in two states. In acute disease they are referred to as planktonic, which means they are free living, single-celled pathogens that live as floating organisms. In chronic disease they become adherent to each other and to a surface, such as the lining of the external ear canal and are referred to as sessile or biofilm infections. These adherent cells produce extracellular polymeric substances that form a slimy matrix around them to protect them from the body's immune system and antibiotics and are recognised at this stage as being 500 times more resistant to antimicrobial agents than the planktonic forms (Costerton et al, 1995). Pseudomonas biofilms have been recognised as a complicating factor in cases of chronic otic infections in man for some time (Bothwell et al, 2003). More recently work by several authors in the veterinary field has shown that when Pseudomonas spp. is present as a secondary infection in cases of canine otitis externa it will produce biofilms in anything from 60% (Pye et al, 2013) to more than 90% (Robinson et al, 2019) of cases. Where biofilm infection with Pseudomonas is present in ear disease it has been shown to be less responsive to topical antibiotics such as polymyxin B, gentamicin and enrofloxacin than non-biofilm infections (Pye et al, 2013).
Why should I recommend cleaning of the dog's ear ?
Ear cleaning is important in the treatment of any case of otitis (Nuttall and Cole, 2004). Cleaning produces a range of benefits for the dog. Cleaners aid in the removal of excessive wax and discharge to make the dog feel more comfortable. They facilitate removal of the mucoid discharge (Figure 3) that prevents penetration of topical drugs into the ear canal. Cleansing allows visualisation of the ear drum which is essential to help decide what topical therapy can be used safely. Many cleaners have antibacterial properties in their own right, others have the ability to potentiate topical antibiotics used in therapy, and some of the newer ear cleaners incorporate ‘biofilm busting’ agents (Nuttall and Cole, 2004; Nuttall and Cole, 2007).
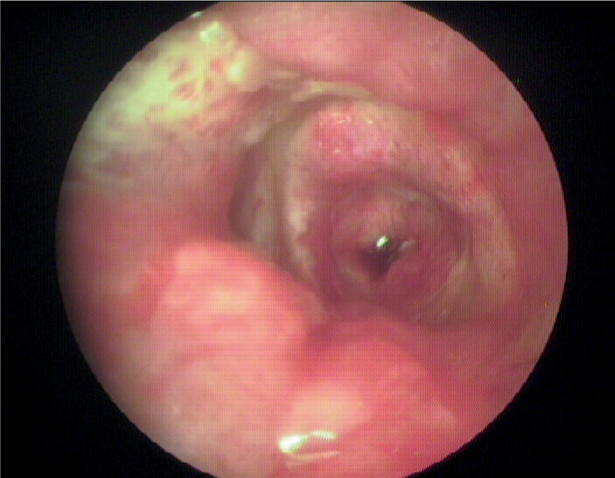
Are some ear cleaners better than others in cases of Pseudomonas spp. infections?
The selection and use of an appropriate ear cleaner is one of the keys to success in all cases of Pseudomonas otitis and nurses play an important part in this. Where the canal is ulcerated and painful, gentle products should be selected to avoid further discomfort to the dog. Although acidic ear cleaners containing components such as acetic and lactic acid have been shown to have antibacterial properties (Steen and Paterson, 2012), they can cause severe pain when they are used in a conscious animal with a painful, ulcerated ear canal. They are therefore best utilised as part of the flushing process in an animal that is admitted to the clinic for an ear flush under general anaesthetic. Cleaners containing Tris ethylenediamine tetra-acetic acid (Tris EDTA) and N-acetyl cysteine have been suggested by some authors as being particularly useful as ingredients of cleaners used in Pseudomonas otitis (Buckley et al, 2013; May et al, 2016).
Why is Tris EDTA recommended as a cleaner in cases of Pseudomonas infection?
Ear cleaners containing Tris EDTA are recommended as part of the therapeutic regimen in Pseudomonas otitis. Tris EDTA has been shown to have anti-Pseudomonas action in its own right (Metry et al, 2012; Chan et al, 2019) as well as having the ability to potentiate the activity of chlorhexidine and both aminoglycoside and fluoroquinolone antibiotics (Wooley et al, 1974, Buckley et al, 2013). Proprietary formulations of Tris EDTA tend to have a neutral or slightly alkaline pH making them less irritating in a delicate ear. Several studies have also shown that Tris EDTA can be used off license if the ear drum is ruptured (Paterson, 2018). Several products are available as ear cleaning solutions that contain Tris EDTA.
What is N-acetyl cysteine and is it useful in cases of Pseudomonas infection?
N-acetyl cysteine comes from the amino acid L-cysteine, it has many uses in human medicine including the therapy of chronic bronchitis and chronic obstructive pulmonary disease where it is thought to act as a mucolytic. It has been used topically in refractory ear infections in man at concentrations of 0.5–2.0% where its mucolytic properties are thought to enhance the penetration of antibiotics in biofilm infections (Choe et al, 2007). It has also been shown to have ‘biofilm busting’ activity (Zhao and Liu, 2010; Dinicola et al, 2014) suggesting it is a useful ear cleaner in cases of chronic Pseudomonas otitis. Several veterinary papers have demonstrated that it has antibacterial properties in its own right (May et al, 2016; Chan et al, 2019). It is available for veterinary use as both a flavoured tablet and a topical ear flush.
Which ear cleaners are considered safe if the ear drum is ruptured?
There are no ear cleaners licensed in veterinary medicine for topical use in otitis media. Owners should therefore always be made aware that topical application of any medication in these circumstances constitutes an off license use of the drug. Numerous reports have demonstrated that aqueous solutions of Tris EDTA have a very low ototoxic potential and is the flush of choice when the ear drum is ruptured in both dogs and cats (Mills et al, 2005). Proprietary solutions containing both Tris EDTA and low concentrations of chlorhexidine (<0.2%) have been used successfully and safely in otitis media in dogs (Paterson, 2018). However while aqueous solutions of Tris EDTA appears to be well tolerated in cats, chlorhexidine even at a concentration as low as 0.05% has been shown to be both cochleotoxic and vestibulotoxic (Igarashi and Suzuki, 1985; Igarashi and Oka, 1988).
What advice should a nurse give a client about cleaning their dog's ears when they have Pseudomonas infection?
The ear canal is ulcerated and painful in the majority of cases of Pseudomonas infection. It is therefore crucially important that dogs do not have topical medication forced on them when their ears are uncomfortable, as this will lead to them becoming ‘ear phobic’. If dogs become fearful of having their ears handled then the application of topical drugs can become impossible, which in turns makes management of the disease very challenging. A useful tip is to advise an owner to give their dog a high value rewards (such as dried liver) to establish a positive emotional state before attempting to apply any ear preparations. Often owners give treats after they have applied cleaner or drops which reinforces negative responses and can have a detrimental effect on subsequent applications of drugs. Owners should always be advised to stop treatment and re-establish a positive emotional state by giving treats if the dog show signs of stress.
How long should a bottle of ear cleaner last and how often should it be replaced?
In order to clean an ear effectively large volumes of fluid need to be applied to flush the canal. The volume applied on each occasion will of course differ between different breeds of dogs. The amount used to clean a Westie's ears will be very much less than that used to clean a New-foundland's ear canal. Generally the author will advise the client to flood the ear with cleaning solution so that they can get an appreciation of the amount that needs to be applied. Little work has been undertaken to assess the efficacy of ear cleaning solutions once they are opened. A single study (Bartlett et al, 2011) has looked at the risk of bacterial contamination of ear cleaning solutions following routine home use. This showed that although bacterial contamination of ear cleaner tips was only 10%, the contamination rate was significantly higher when Tris EDTA was an ingredient. The author would therefore recommend that a new bottle of ear cleaner is dispensed at the start of every course of therapy and that owners should be discouraged from using ear cleaning solutions that have been used on previous occasions.
What part can the nurse play in preventing the recurrence of Pseudomonas otitis?
Pseudomonas infection is a secondary cause of otitis. Where the disease is recurrent, the infection needs to be addressed but the primary underlying cause needs to be identified and managed. Common underlying causes of Pseudomonas otitis include allergy, endocrine disease, immune-mediated disease and neoplasia. The aim in all cases should be to resolve infection aiming for an end point of normal ear canal cytology, manage underlying primary causes, and reverse any chronic change within the ear canal. Once this has been achieved regular reassessments should be undertaken. These can easily be performed by a nurse in a dedicated otology clinic. Rechecks should include taking a history from the client to ensure the dog has been stable between rechecks, an otoscopic examination of the ear canal, and cytology of any discharge to look for signs of an inflammatory infiltrate together with increased numbers of micro-organisms.
Conclusion
Pseudomonas species are Gram-negative, rod-shaped bacteria. P. aeruginosa is an opportunistic pathogen commonly implicated as a cause of otitis externa/media in the dog. When inflammatory change occurs in the ear due to primary underlying causes, Pseudomonas overgrowth can occur leading to secondary infection. In chronic disease biofilm formation is a common sequela. Cytology of the otic discharge together with culture and susceptibility are important to establish the type of infection and the most appropriate antibiotic therapy. Treatment should include thorough cleaning of the ear. Nurses play a key role in helping with the initial cleaning and instruction of owners on appropriate protocols to treat their pet. Acidic cleaners have good antibacterial properties but are often not tolerated if the ear canal is ulcerated and painful. Tris EDTA based cleaners help to potentiate topical antibiotics and are very safe if the ear drum is damaged. N-acetyl cysteine has biofilm busting activity and can be useful employed as part of a treatment regimen. Nurses can play an invaluable role in the reassessment of cases through dedicated otology clinics.
KEY POINTS
- Pseudomonas spp., particularly Pseudomonas aeruginosa, are opportunistic pathogens commonly found causing secondary infection in otitis externa/media in the dog.
- Otic discharge cytology should be followed by culture and sensitivity testing whenever bacterial rods are detected causing infection, to enable choice of appropriate antibiotics.
- Biofilm formation is common in chronic disease.
- Treatment includes thorough cleaning of the ear in addition to use of appropriate antibiotics.
- Acidic ear cleaners, while having good antibacterial properties, often are not tolerated by dogs with ulcerated painful ear canals.
- Cleaners based on Tris ethylenediamine tetra-acetic acid (Tris EDTA) help to potentiate topical antibiotics, may be less painful, and are safe to use if the ear drum is damaged.
- Agents with ‘biofilm busting’ activity include Tris EDTA, honey, N-acetyl cysteine and silver.


