Toxocara canis and Toxocara cati are both ubiquitous, every day parasites that we take for granted and there may be a tendency to believe that we know it all. However, recent work, for example, on the pathology caused by T. cati in the lungs of cats and the body of data that exists on the effects of this parasite on humans, have produced sufficient new data to merit a review of what is known about this enigmatic parasite. This review does not go systematically through all aspects of the infection and these details can be found on, for example, the www.esccapuk.org.uk website.
Update on the lifecycle of Toxocara species
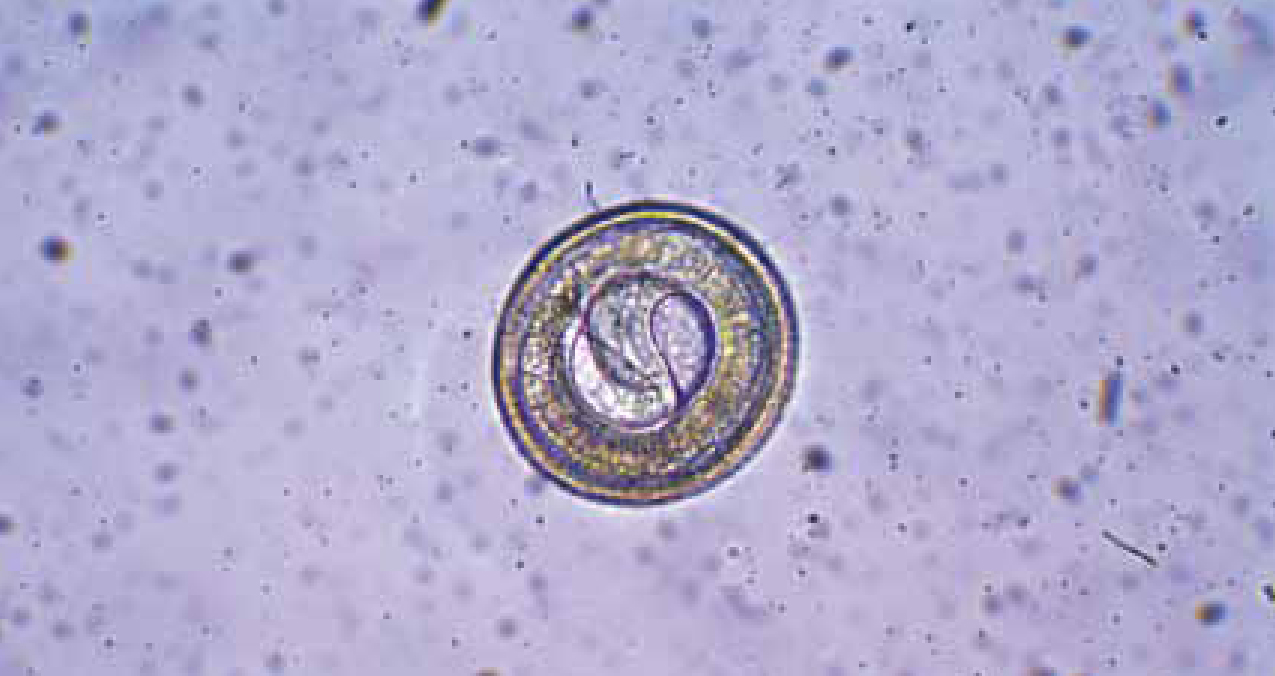
Female Toxocara spp. are prolific egg layers, with each worm able to lay up to 20 000 eggs. Eggs are passed into the environment within faeces and take a period of time to develop within the egg, depending on environmental conditions. Traditionally it was believed that the second stage larva was the infectious stage that remained protected by the egg shell until ingested by a host. Recent work has demonstrated that there are two moults of the larva within the egg, hence the infectious larva within the egg is actually third stage (Figure 1) (Schnieder et al, 2011).
Contribution to environmental contamination with Toxocara spp. in an urban area, such as Bristol, is likely to be largely due to canine faeces (Morgan et al, 2013). If the bulk (90% or more) of dog faeces are collected and disposed of then fox faeces become the main source of environmental contamination, with cats contributing less based on estimations of the cat population, prevalence and intensity of infection in the three definitive host species (Morgan et al, 2013).
Effects of Toxocara species in animals
Larval migration
Within either a definitive host (dog or cat, depending on species) or paratenic host (other animals including humans), larvae hatch within a few hours of ingestion within the duodenum and the larvae migrate out of the intestine and travel via the portal vein to the liver and subsequently to the heart and then the lungs.
In the definitive host larvae may leave the circulatory system in the lungs to be coughed up and swallowed, thus returning to the intestine about a week after ingestion, where they complete their development to adult worms (Figure 2). Alternatively, larvae on reaching the lungs may be further distributed in the circulation to a variety of tissues, resulting in somatic migration. Larvae can survive for 10 years in a variety of tissues.

Effects on the paratenic host
Somatic migration occurs in paratenic hosts where favoured migration routes vary according to host species (Strube et al, 2013). For example, in rats a proportion of larvae migrate to the cerebellum which can result in a reduction in learning ability in affected rats accompanied by random wandering and a reduction in wariness. It has been suggested that this type of behaviour causes an increase in the likelihood of the animal being predated, thus perpetuating the life cycle (Chieffi et al, 2009). Holland and Hamilton (2013) have described a mouse model to examine this relationship between host and neurotrophically migrating larvae further. They suggest that cytokines released as the larvae migrate may be associated with memory loss. Further work in this area will permit a better understanding of the pathological and behavioural effects of larvae in paratenic hosts including humans.
Effects on the definitive host
In the cat, larvae reaching the lungs undergo local migration before continuing with their migration and development. Ray Dillon and colleagues (2013) conducted a study focussing on the changes that may be caused by T. cati larval migration in the lungs. Following administration of 10 000 larvae per cat in doses of 2000 larvae over a 6 week period (which represents a large number of larvae, probably more than most cats encounter over a short time period), no clinical signs were observed. However a large number of eosinophils were observed in both blood samples and bronchoalveolar fluid 35 days after the first infection, although there was no clear association between the number of eosinophils in the blood and bronchoalveolar fluid. Radiographs taken on day 64 after the first infection showed increased peribronchiolar opacity. On computed tomography changes were seen particularly in the caudal lung lobes. Whereas the lungs of uninfected cats grew over the period of the study, those of infected cats reduced by almost 8% over 9 weeks following the first infection. This study indicates that T. cati infection should be considered as a differential diagnosis when lung disease is identified, with changes seen as early as 11 days after infection. It is notable that, since the pre-patent period of T. cati is approximately 56 days, signs of lung disease may be seen weeks before infection can be demonstrated by the presence of eggs in the faeces.
While the study by Ray Dillon et al (2013) was able to utilise modern diagnostic techniques to examine the effect of migrating larvae on the host, there have been previous studies to examine the effect of administration of larvae to the definitive host. For example, Hayden and van Kruiningen (1975) examined the effect of 50 000 T. canis larvae administered to eight puppies that had previously been infected with T. canis when younger. This infection resulted in eosinophilic gastroenteritis and focal lesions in a variety of tissues including the liver and lungs.
Retinitis caused by Toxocara spp larvae is generally thought of as a consequence of human infection. However it has been recognised for some time that retinitis in dogs can be caused by migrating T. canis larvae; 39% of 1448 working sheep dogs were found to have multifocal retinal disease confirmed on histopathology as associated with migration of Toxocara spp larvae in a number of the dogs (Hughes et al, 1987).
Although adult ascarids in the definitive host are capable of causing disease, this review illustrates that migrating larvae are also capable of causing a variety of pathological consequences.
Effects of Toxocara species infection in humans
Toxocara spp. infection in humans has been classified into three syndromes depending partly on the migration of the larvae and partly on symptoms observed. Visceral larva migrans (VLM) is seen as a large number of larvae migrate through organs. This is rarely seen in countries such as the UK where the larval challenge is likely to be too low to cause VLM. Ocular larva migrans (OLM) results from migration of one or more larvae across the retina of the eye. There are few good studies to evaluate the prevalence of OLM, and so there is a lack of data on the number of affected individuals. One well conducted study carried out in Ireland estimated six per 100 000 schoolchildren were affected (Good et al, 2004).
Infection in some individuals is associated with non-specific signs such as headache or stomach ache, associated with so-called covert toxocarosis. Covert toxocarosis is also implicated in numerous reports of correlations between seropositivity and symptoms such as asthma, chronic coughing (Bede et al, 2008) or findings such as decreased lung function (Walsh, 2010) or cognitive function (Walsh and Haseeb, 2012). While the quality of these types of studies may be hampered by small sample size and a variety of clinical presentations, the latter two references were conclusions based on a large scale survey of US citizens and was carefully assessed by the authors for possible confounding factors before they made their conclusions.
Diagnosis of Toxocara infection in humans
Diagnosis of toxocarosis in humans depends on serology, clinical signs and the exclusion of other diagnoses. In covert toxocarosis in particular, symptoms such as headache are non specific, hence difficult to link conclusively to positive serology, as a proportion of the population are seropositive without any clinical signs. In such cases confirmation of a diagnosis is based on exclusion of other differential diagnoses (Fillaux and Magnaval, 2013) Diagnosis is complicated in some cases of OLM by an absence of seropositivity. Diagnosis is also complicated by an absence of a standardised test system and an accepted system capable of distinguishing T. canis from T. cati infections (Fillaux and Magnaval, 2013). One report of a system capable of distinguishing the two infections suggests that up to one third of Toxocara spp related disease in humans is related to T. cati infection, hence it is a research priority to develop a widely accepted test that is capable of accurately distinguishing the two infections.
Prevention of human infection
Avoidance of infection is possible by measures such as educating children to wash their hands before eating, removing dog faeces from public places (Figures 3 and 4), covering sand pits, and appropriate anthelmintic treatment of dogs and cats (ESCCAP, 2010).


Conclusion
Despite the ubiquitous nature of Toxocara spp, understanding of this parasite and its interactions with its hosts remains incomplete. Recent work has demonstrated that third stage larvae infect and migrate in both paratenic and definitive hosts, where behavioural changes and lung disease, respectively, may be observed as a consequence. Larval migration in humans is linked to vision impairment, asthma, lung function and cognitive function impairment. Diagnosis in humans remains hampered by the constraints of the current tests.

