Feline caliciviruses (FCVs) are recognized as an important, highly infectious pathogen of cats, with a widespread distribution in the feline population (Radford et al, 2007; Patel and Heldens, 2009). FCV was first described in 1957 by Fastier and usually causes mild, acute, and self-limiting upper respiratory tract disease that causes frequent nasal and ocular discharge (Figure 1) (Fastier, 1957; Radford et al, 2007).
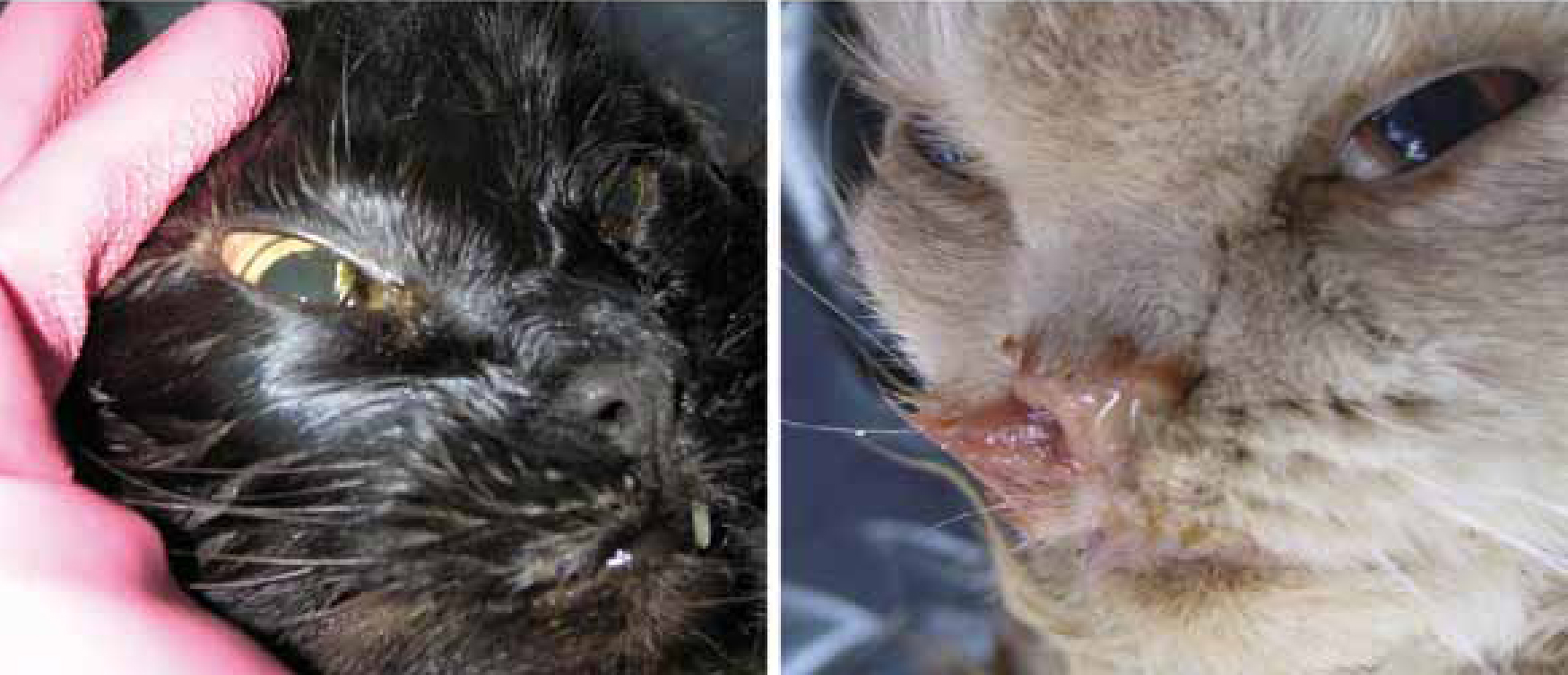
Other commonly found signs are oral vesicles/ulcers, conjunctivitis, rhinitis, tracheitis, pneumonia, fever, anorexia and lethargy. Morbidity is high, mortality may achieve 30% in very young kittens and recovery is followed by a prolonged carrier state. Aerosols and fomites are usually the transmission vehicle and the virus is often carried to susceptible cats by human handlers, including veterinary staff. The incubation period is 2 to 6 days and the virus is shed in large numbers by infected cats (Radford et al, 2007). Recuperating cats may continue to shed the virus for several months and stress is known to precipitate the recrudescence of the disease and therefore additional shedding (Murphy et al, 1999; Radford et al, 2007).
Although all Felidae are apparently vulnerable, natural infection has been reported only in domestic cats and cheetahs. The death of valuable kittens and the costs of providing supportive treatment for sick animals have caused substantial economic losses, which has helped in raising awareness of FCV. About 50% of cats with clinical signs of acute upper respiratory disease are shedding the virus, but by the age of 1 year almost all have developed antibodies to it, therefore, clinical disease is rare in cats over this age (Murphy et al, 1999).
The FCV belongs to the Caliciviridae family which includes four distinct genera: Lagovirus, Sapovirus, Norovirus and Vesivirus, the last of which comprises FCV and several other viruses of veterinary importance, including vesicular exanthema of swine viruses, San Miguel sea lion viruses and cetacean calicivirus (Murphy et al, 1999).
During replication RNA viruses, such as FCV, produce a significant number of errors in the viral synthesis. Genetic variation in RNA viruses is also expected as a result of viral recombination (Rao and Hall, 1993). The combined action of both these mechanisms accounts for high genome flexibility in FCV, thus allowing the virus to rapidly adjust to environmental selection pressures (Radford et al, 2007). In the light of FCV adaptational potential, important implications for clinical disease are likely to occur and extensive knowledge on viral inactivation is crucial in tackling the possibility of the emergence of new FCV strains. Indeed, the emergence of the human 2009 pandemic of Influenza A virus subtype H1N1, a recombination of several strains (Smith et al, 2009), has shown that there is reason to be apprehensive.
FCV, an emerging virus
Like all Caliciviruses, FCV mutates readily in nature, and this has resulted in the recent emergence of a highly virulent haemorrhagic variant of FCV (Pedersen et al, 2000; Ossiboff et al, 2007; Radford et al, 2007), which is spreading rapidly (Hurley et al, 2004; Pesavento et al, 2004). The shedding of this new variant (named virulent systemic FCV) can occur for up to 15 days through urine, saliva, respiratory and ocular secretions, and can be conveyed by aerosols, enterically or on fomites (Patel and Heldens, 2009).
Primary infection by virulent systemic FCV is often in the upper respiratory tract and/or oral mucosa with an incubation period of 2–3 days, resulting in epithelial cell necrosis with external nares and oral mucosa ulceration or vesicle formation, usually with a fatal outcome (Patel and Heldens, 2009).
Unlike FCV, which has a worldwide distribution, the virulent systemic strain was until recently limited to the USA and the UK (Radford et al, 2007), however, an outbreak of nosocomial origin has been reported in a veterinary teaching hospital in France, which lead to the death of over 50% of the infected cats (aged 1–12 years) (Reynolds et al, 2009). While the virulent systemic FCV is spreading and likely to become more prevalent (Patel and Heldens, 2009; Reynolds et al, 2009), the general increase in respiratory viral infections is simultaneously being reported, probably due to growing animal populations and increasing population densities in many settings (Murphy et al, 1999). In addition, nosocomial transmission of viruses are reported to be highest in companion animal practices, when compared with other veterinary facilities, and are a well-known contributing factor to the spread of disease and a direct cause of increased veterinary medical costs (Scott, 1980;Brown, 1981; Murphy et al, 1999). Advances in FCV inactivation are, therefore, urgent and will prove to be a major asset in controlling the potentially emergent virulent systemic strains.
FCV inactivation
The high morbidity, the cost-of-illness and the fact that no drug is currently available to treat this disease justifies per se the choice of FCV as a target for viral inactivation research. Moreover, in light of the detection of new emergent strains with increased pathogenicity, veterinary staff and pet owners are highlighting concerns about FCV control. As a prophylactic measure, the use of surface disinfectants has been a central tool in FCV control but, to the authors' knowledge, to date, only a few FCV inactivation studies have been published. These studies date back as far as 30 years and looked at older virucidal chemicals as active ingredients, often harmful for the user and surfaces.
In 1980, a study by Scott et al on 35 commercial products, mainly used in veterinary medicine, revealed that only 11 were virucidal for FCV, after an exposure time of 10 minutes. Aiming at the simultaneous inactivation of feline viral rhinotracheitis, feline calicivirus and feline panleukopenia virus, 0.175% sodium hypochlorite solution was the most effective and practical broad-spectrum virucidal product (Table 1). In 1995, Kennedy et al tested the virucidal activity of several disinfectants containing newer generation quaternary ammonium compounds, but none was able to completely inactivate FCV. In this study, sodium hypochlorite was found to be the only disinfectant to completely inactivate all viruses (Kennedy et al, 1995). More recently, Eleraky etal (2002), conscious of the deleterious effects of sodium hypochlorite, compared the virucidal efficacy of four available disinfectants marketed for general use in veterinary clinics (chlorine dioxide, potassium peroxymonosulfate, a quaternary ammonium compound, and citricidal) and included 3% sodium hypochlorite as a positive control. All commercial brands claimed to possess broad antimicrobial specificity but the quaternary ammonium compound and the citricidal were found to be ineffective against FCV (Table 1).
Table 1. Effective active ingredients concentrations (and respective contact times) against FCV, under different surface types.
| Active ingredient | Concentration | Contact times | Surface types | References |
|---|---|---|---|---|
| Sodium hypochlorite | 0.175% | 10 min | VS | Scott et al, 1980 |
| Sodium hypochlorite | 3% | 10 min | VS | Eleraky et al, 2002 |
| Chlorine dioxide (commercial) | Manufacturers recommendation | 10 min | VS | Eleraky et al, 2002 |
| Potassium peroxymonosulfate (commercial) | Manufacturers recommendation | 10 min | VS | Eleraky et al, 2002 |
| Activated 2.6% glutaraldehyde (commercial) | Undiluted | 1, 5, 10 min | Fabrics | Malik et al, 2006 |
| 55 to 60% formic acid (commercial) | 0.5% | 15 min | VS | Poschetto et al, 2007 |
| 4% | 15 min | VS + 40% Fetal Bovine Serum † | ||
| 4% | 15 min | VS + 25% Feces † | ||
| 20 to 25% glutaraldehyde (commercial) | 0.1% | 15 min | VS | Poschetto et al, 2007 |
| 0.1% | 15 min | VS + 40% Fetal Bovine Serum † | ||
| 0.1% | 15 min | VS + 25% Feces † | ||
| 12% sodium hypochlorite (commercial) | 0.5% | 15 min | VS | Poschetto et al, 2007 |
| 0.5% | 15 min | VS + 40% Fetal Bovine Serum † | ||
| 0.75% | 15 min | VS + 25% Feces † | ||
| 14 to 16% peracetic acid, 22 to 24% | 0.1% | 15 min | VS | Poschetto et al, 2007 |
| hydrogen peroxide, <15% acetic acid (commercial) | 0.1% | 15 min | VS + 40% Fetal Bovine Serum †, | |
| 1% | 60 min | VS + 25% Feces † | ||
| 1-propanol | 70% | 30 sec | Fingertips | Gehrke et al, 2004 |
| Ethanol | 70% | 30 sec | Fingertips | Gehrke et al, 2004 |
| Ethanol | 99.5% | 30 sec | Fingertips | Lages et al, 2008 |
| Povidone-iodine | 10% | 30 sec | Fingertips | Lages et al, 2008 |
| Ethanol + polyquaternium polymer + organic acid | manufacturer's recommendation | 30 sec | Fingertips | Macinga et al, 2008 |
VS – Virus suspension;
†Fetal Bovine Serum and Feces were introduced to simulate organic load.
The immunoprophylaxis contenda
Despite the scarcity of FCV inactivation studies, vaccination against FCV has been available for some years and has effectively reduced the incidence of clinical disease (Radford et al, 2007). It is therefore tempting to conclude that this might be the reason why research on FCV inactivation, with the specific goal of FCV infection control, has diminished in recent years. However, the vaccines do not prevent infection and vaccinated cats can still become persistently infected. In addition, FCV strain variability means that vaccinated cats are not protected against all strains equally (Radford et al, 2007) and in the light of the potential emergence of systemic virulent strains, more research needs to be performed.
FCV as a surrogate for norovirus: data for veterinary nursing
Variation seems to be a characteristic across all Caliciviridae family especially within the Norovirus genus (Patel et al, 2009). Noroviruses are known as the most common cause of epidemic gastroenteritis in people of all age groups, accounting for more than 90% of viral gastroenteritis and 50% of all cause outbreaks worldwide (Patel et al, 2009; Mesquita et al, 2010). As the public health impact of norovirus infections is increasingly being recognized, there is an urgent need to assess the virucidal efficacy of hand, surface and instrument disinfectants, in order to disrupt chains of infection in hospitals and other medical areas. Moreover, as no norovirus drug or vaccine is currently available, the discovery of virucidal disinfectants has been gathering increasing attention from scientists. Due to the absence of a cell culture system or animal model supporting the replication of noroviruses (Patel et al, 2009), it has been necessary to use a surrogate virus with similar properties and there have been numerous studies using FCV as a surrogate (Steinmann, 2004; Cannon et al, 2006).
FCV, which can be readily grown and assayed in cell culture (Cannon et al, 2006), has been introduced as a surrogate virus for studying the stability of norovirus; in 1993 Jiang et al reported that FCV shares common biochemical properties, similar sequence and genome organization with norovirus. Five years later, in order to investigate heat inactivation of noroviruses in shellfish, Slomka and Appleton (1998) introduced FCV as a model. Since then, several experiments on the inactivation behaviour of various surface disinfectants have used FCV as a surrogate (Doultree et al, 1999; Gulati et al, 2001; Lopman et al, 2002; Bidawid et al, 2004; Duizer et al, 2004), to monitor norovirus survival. The information obtained through these experiments is spread across several norovirus-themed areas, such as environmental or clinical (Lopman et al, 2002; Malik et al, 2006; Hudson et al, 2007; Lages et al, 2008) and food virology (Slomka and Appleton, 1998; Butot et al, 2008; Lamhoujeb et al, 2008). All this information is easily available and deserves to be compiled for use by the veterinary nursing community in FCV infection control.
Which disinfectant can be used?
The search for an appropriate disinfectant with a strong norovirus virucidal activity has led to a considerable number of studies being undertaken that in turn have generated a huge amount of data on FCV inactivation. These findings are useful to veterinary nurses who can improve their practice and minimize FCV transmission.
Textiles
In an effort to evaluate the efficacy of disinfectants on textiles contaminated with noroviruses, Malik et al (2006) dried FCV on fabrics and carpets and treated them with disinfectants. Three types of fabrics (100% cotton, 100% polyester and a 35:65 cotton/polyester blend) and four types of carpet (olefin, polyester, nylon and an 85:15 olefin/nylon blend) were selected as representative of fabrics and carpets found in healthcare facilities. Among five commercially available disinfectants, an activated 2.6% dialdehyde-based product was found to be the most effective disinfectant on all types of fabric and carpet, inactivating more than 99.99% of the virus in 1 to 10 minutes (Table 1). In addition, the authors concluded that general effectiveness increases with increasing exposure time, from 1 to 10 minutes, and that disinfection of carpets was more difficult than disinfection of fabrics; 100% polyester being the least amenable to disinfection (Malik et al, 2006).
Field-like conditions
In an experiment, Poschetto et al (2007) evaluated the sensitivity of FCV to chemical disinfection, under field-like conditions (40% fetal bovine serum or 25% fecal suspension was added to simulate the organic load that is normally encountered and that usually diminishes the virucidal activity of disinfectants) and, in general, disinfectant efficacy was strongly reduced by the organic load. Despite spiking with organic load to reproduce norovirus field conditions, all disinfectants were found to be effective against FCV. It is worth noting that, when spiking with fecal material, the inactivation time increased from 15 to 60 minutes, and the concentration increased from 0.1 to 1% (Table 1).
Hands
The hands of the veterinary staff are considered to be another important vehicle in FCV transmission (Murphy et al, 1999). In a norovirus-themed study, aware of hand disinfection as an important procedure in preventing the person-to-person transmission, a research group investigated the virucidal efficacy of nine hand sanitizers (four alcohol-based sanitizers, three non alcohol-based sanitizers and two triclosan-containing antimicrobial liquid soaps) against FCV on artificially contaminated fingertips (Lages et al, 2008). Among alcohol-based sanitizers, 99.5% ethanol was more effective than 62% ethanol, 70% isopropanol or 91% isopropanol. The virucidal activity in antiseptics containing 10% povidone-iodine was the highest in the test. Results also revealed that triclosan-containing antimicrobial soaps or alcohol-based hand rubs may prove to be inadequate in preventing FCV transmission (Lages et al, 2008). In another study, Gehrke et al (2004) concluded that ethanol and 1-propanol-based solutions, with high alcohol content, appeared to be the most effective against FCV and possessed sufficient inactivating properties to be recommended as hand sanitizers (Table 1). Other researchers tested a new ethanol-based hand sanitizer, containing a synergistic blend of polyquaternium polymer and organic acid (Macinga et al, 2008). When compared with a benchmark hand sanitizer (62% ethanol) and a 75% ethanol reference solution, the synergistic blend demonstrated higher virucidal activity (Macinga et al, 2008).
Other techniques
The search for a suitable norovirus inactivation technique has also revealed new disinfection procedures that may prove to be useful in FCV inactivation. In an attempt to provide information on norovirus inactivation by ozone gas in conditions relevant to healthcare, a research group has reported that FCV can be inactivated by exposure to ozone gas, from a portable commercial generator, in settings such as hotel rooms, cruise ship cabins and healthcare facilities (Hudson et al, 2007). Another research group provided information on the effectiveness of UV radiation on emerging viral enteric pathogens present in water (with FCV as a surrogate for human noroviruses), having achieved 99.99% FCV inactivation with a regular easily available 8-W low-pressure mercury vapour germicidal lamp (Thurston-Enriquez et al, 2003).
Conclusions
Healthcare-associated infections are a major problem for patient safety (Murphy et al, 1999). Consequently, their surveillance and prevention deserves the full attention of veterinary nursing professionals and must be a top priority for settings committed to making animal health care safer. Infection control of FCV is a critical component of safe, quality health management, and needs to be addressed with every tool available, in order to provide the best animal care possible. In general, the latest research on norovirus inactivation seems to suggest that the use of glutaraldehyde-based disinfectants on surfaces, as well as ethanol and 1-propanol-based solutions with high alcohol content on hands, can be an effective tool in the control of FCV, thus being worthy of further research. Novel techniques such as inactivation by ozone gas exposure or UV irradiation may also prove to be valuable in FCV control.
The contact time of disinfectants in the experimental conditions is often of 10 minutes, nevertheless, this contact period seems to be longer than what typically occurs on a day-to-day basis, as in cage cleaning. Moreover, the organic load (blood and faeces) is likely to be present in the veterinary practice; therefore the organic load has to be considered when determining the contact time.
The correct use of state-of-the-art disinfectants, together with adequate disease control in different situations, such as clinics, the pet's home environment, boarding catteries, shelter facilities and breeding catteries, can be effective in FCV control.
Key Points
- Feline calicivirus (FCV) is a highly infectious and widespread pathogen of cats.
- A highly virulent and fatal FCV strain is emerging; therefore advances in FCV inactivation are urgent.
- Several experiments on the inactivation behaviour of various surface disinfectants have used FCV as a surrogate for norovirus, therefore data on FCV inactivation are available.
- Norovirus inactivation research seems to suggest the use of glutaraldehyde-based disinfectants on surfaces, as well as ethanol and 1-propanol-based solutions with high alcohol content on hands, as effective tools in FCV control.

