The veterinary nurse's (VNs) main aim of surgical site preparation is to reduce the number of bacteria and bacterial activity present at the surgical site without damaging the patient's skin (Wilson et al, 2011). It has been documented by McHugh (2012) that horses are a great source of contamination with both endogenous and exogenous microorganisms. Exogenous microorganisms are the biggest source of contamination into the surgical field, thus the VN must aim to reduce this number to a minimum. Equine skin is the most common source of contamination, as the skin can never be fully disinfected, however the bacterial count should be reduced to as low a level as possible. It is the VN's responsibility to ensure that the skin is disinfected and bacterial numbers are minimal by using evidence-based protocols, to reduce the risk of surgical site infections (SSIs) (Adams, 2012).
The skin has two distinct bacterial populations. Resident bacteria which occupy the deeper adnexal structures around hair follicles and sebaceous glands, these are minimally pathogenic, but have the potential to cause SSIs. Transient bacteria represent surface bacteria that are accessible to the actions of antiseptics, these bacteria do not normally colonise the skin, and are gained via contact with people, animals or the environment. The transient population of bacteria are the target for the antiseptic solutions. Adams et al (2010) investigated bacterial load on the skin of hospitalised horses and found that Staphylococcus spp. is the most commonly isolated bacteria, thus VNs must select an appropriate disinfectant to reduce the staphylococcus population to a minimum (Bowers, 2012), as bacteria cannot be completely removed due to 20% of surface microorganisms being inaccessible to routine disinfection because they are protected by hair follicles, crevices, and lipids (Henning et al, 2001).
Preoperative grooming
VNs should groom their patients preoperatively to reduce contamination, to reduce excess hair, skin scales and external parasites and subsequently reduce the degree of contamination in theatre and subsequently the surgical site (King, 2014). Care should be taken to ensure that distal limbs are clean, especially if orthopaedic surgery is going to be undertaken, as this region is more likely to have a higher bacterial load as transient bacterial flora of the skin are influenced by the amount of dirt and exudates, the length of hair and the amount of moisture present in that area of skin (Wilson et al, 2011). Also if the patient is a native breed, for example a Welsh Cob or Highland, their distal limbs are likely to harbour increased bacteria due to having extra feathers on their legs.
If patients are grossly contaminated, preoperative bathing should be performed; this task of preoperative bathing is usually delegated to the owner prior to admission, however not all owners are compliant, especially if they do not have warm water washing facilities, thus this procedure should be undertaken in the hospital by VNs. This ensures compliance and allows the VN to check the skin's integrity. However, pre-operative bathing has been shown to temporarily increase exfoliation of skin squames, increase hair shedding and produce rebound population of resident microbes, thus leading to some controversy regarding pre-operative bathing (Gasson, 2010; Roberts, 2013). VNs should, therefore, make a decision prior to surgery, as to whether the patient requires preoperative bathing, based on their degree of contamination.
Hair removal
Hair is perceived to be associated with lack of cleanliness and hair removal is thought to reduce the risk of SSIs, however the type of hair removal method also affects SSIs. VNs should not use a hand held razor or scalpel blade for hair removal as this can cause microabrasion of the surface and traction on the hairs which causes expulsion of follicular contents onto the skin surface, resulting in skin surface contamination from resident bacterial populations, which has been shown to be associated with a ten-fold increase in SSIs (Bowers, 2012). Depilatory creams should not be used as a method of hair removal in practice, as these chemicals dissolve the hair, and cutaneous reactions can occur (Baines et al, 2012).
Clippers have been advocated by a range of authors such as Gasson (2010) and Bowers (2012) for hair removal as clipping is associated with the lowest incidence of SSIs, however SSIs are increased by poor clipping technique, as trauma to the skin can lead to skin abrasions and colonisation by resident microbes. To reduce this risk, VNs must ensure a careful clipping technique is employed and well serviced well lubricated clippers with size 40 blades are used.
Hair should not be removed more than 12 hours preoperatively as the rate of SSIs is proportional to the duration of time the hair was removed (Joyce, 2006). Ideally clipping should be performed once the patient is anaesthetised to reduce bacterial colonisation, thus reducing the risk of SSIs (McMillan, 2014) (Figure 1). However there are limitations for clipping post induction, as equine patients are relatively difficult to move compared with the ease of moving a small animal into theatre after clipping. Also, a preparation area may not be available in equine surgery, thus equine patients are often clipped prior to induction (Packer and Devaney, 2010). In addition, post induction clipping increases the length of anaesthetic time — increased anaesthetic time is a contributing factor to SSIs, for every minute of additional anaesthesia time there is a 0.5% increased risk of SSIs (Beal et al, 2000). Therefore practices should formulate guidance for VNs regarding preoperative clipping, taking into account, the hospital facilities, patient's health status and surgery required.
Clippers should be segregated between ‘clean’ and ‘dirty’ procedures to reduce the risk of spreading infection between patients. Following use, clippers and blades should be cleaned. The blades can be cleaned with a tooth brush to prevent hair from being caught between blades and subsequently preventing a close contact clip. Once cleaned all blades should be inspected to ensure that they are fit for use (Figure 2). VNs should consider sterilising clipper blades between patients, as repeated use of clipper blades without sterilisation results in higher levels of bacterial contamination of blades than when blades are sterilised. If clipper blades are not sterilised between patients they should be disinfected to prevent cross contamination (Wesse, 2008; Beco et al, 2013).

Patients should be clipped outside of theatre, preferably in a preparation room to prevent hair particles contaminating the surgical environment (Shellim, 2007; Wesse, 2008; Bowers, 2012), however in human medicine there is no research to indicate whether the place of hair removal affects SSIs. In addition, it is unclear whether human recommendations can or should be directly related to the equine veterinary field as horses live in a contaminated environment and their hair moults(Tanner et al, 2008). The recommended clipping margin around the proposed incision site should be approximately 15–20 cm, this sized area should reduce the likelihood of hair contaminating the surgical incision (Bowers, 2012). The clipped hair should be removed using a vacuum to reduce the likelihood of hair becoming a source of contamination, and following surgery the vacuum's filter should also be cleaned and emptied.
Antiseptic solution
There are several problems that could be encountered in practice when preparing skin for surgery, this includes contamination of the preparation solution, inadequate initial cleaning of the surgical site, failure to prepare a large enough area, inadequate antiseptic contact time and contamination of the area during or after preparation.
Chlorhexidine gluconate and povidone iodine are the most commonly used skin antiseptics in practice. Povidone iodine is mainly used on areas of the head, as chlorhexidine gluconate should not be used in this region as it is harmful to the corneal epithelium and results in neurosensory deafness following contact with the middle or inner ear (Archer, 2013). An equine-based study by Wilson et al (2011) demonstrated that chlorhexidine gluconate resulted in greater bacterial reduction after the cleansing scrub than occurred with povidine iodine, this could be due to chlorhexidine gluconate being minimally inhibited by organic material (Southwood, 2015). Chlorhexidine gluconate also has a residual effect which lasts up to 6 hours, this residual effect allows surgery to be completed and a bandage applied prior to the patient returning to its stable (Rippingale and Fisk, 2013).
Correct concentrations of chlorhexidine gluconate are not always used in practice and 62% of small animal practices do not measure concentrations of skin disinfectant (Evans et al, 2009). To prevent this, the VN should use a set dilution ratio of 50:50 chlorhexidine gluconate to warm water (Roberts, 2013).
In human surgical site preparation, both mechanical and chemical methods are required for efficient removal of resident microorganisms. The mechanical process includes applying a skin antiseptic solution with adequate friction to ensure that all cracks and fissures in the skin are adequately coated (Scowcroft, 2012), however, it has been suggested that mechanical scrubbing is not necessarily advantageous, as it can result in an increased number of surface bacteria, perhaps caused by the release of resident skin bacteria (Davids et al, 2015).
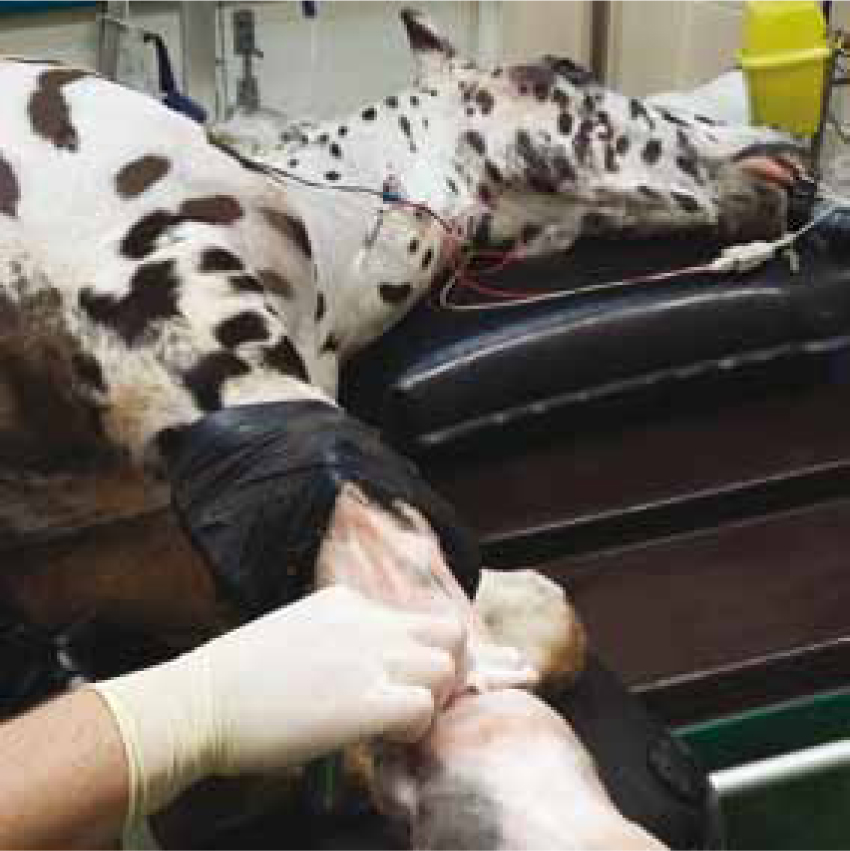
Skin antiseptic preparation should be divided into the initial preparation and then final preparation, however both preparations should use the same antiseptic solution. The initial preparation is performed outside of theatre, using swabs and wearing gloves (Figure 3), this is usually performed in a preparation room which is not available in all theatre suite designs. VNs should prepare the surgical site using a ‘back and forward motion’ concentrating on the incision site (McMillan, 2014).
Non-lint producing gauze swabs should be used as cotton wool is prone to leaving fibres, which could enter the incision and act as a foreign body or be a means of transport for microbes (Roberts, 2013). VNs should only apply gentle pressure when using gauze swabs, as too much pressure could lead to skin irritation and bacteria from within the hair follicle being bought to the skin surface (Shellim, 2007). An additional benefit of using gauze swabs includes the ability of the VN to check the cleanliness of the skin by wiping with a swab and checking the white swab for gross contamination (Southwood, 2015).
The final sterile skin preparation should be performed in theatre by the VN, using the same antiseptic solution as used previously diluted in either sterile water or saline, applied with sterile gauze swabs and either sterile sponge holding forceps or sterile gloves to prevent contamination from the VNs hands.
To prevent contamination of the antiseptic solution, chloraprep® (Invicta Animal Health), which is a 2% chlorohexidine gluconate and 70% isopropyl alcohol, could be used. The applicator stores the sterile solution in an ampoule prior to application and is clicked to release the solution. Chloraprep® has additional benefits such as the foam sponge, which prevents splashing or pooling of solution and prevents excessive friction and subsequent skin irritation. Chloraprep® also provides persistent antimicrobial activity, thus its antimicrobial action will continue following first incision reducing the likelihood of SSIs; chloraprep also prevents contamination of the surgical site from the VNs hands (Bourel et al, 2013).
Rinsing agent
Traditionally alcohol-based preparations, such as 70% isopropyl alcohol, have been applied to surgical sites following a disinfectant scrub — as alcohol is a rinsing agent, this would result in similar, if not greater sterility than sterile saline (Davids et al, 2015). However, in an equine-based study, Wilson et al (2011) demonstrated that 3 hours after skin preparation, chlorhexidine rinsed with alcohol had higher colony forming units, than chlorhexidine rinsed with sterile saline. So in equine patients it is suggested to rinse the surgical site with saline.
Hands
VNs' hands are a major source of infection and can be an influential factor in patients having SSIs. VNs should ensure good hand hygiene, either proper handwashing or the use of alcohol-based hand rubs (following the world health organisation (WHO) technique), before and after patient contact (Wesse, 2008). To reduce the likelihood of infection further, VNs should wear gloves when preparing the surgical site. Wearing gloves prevents contaminating the patient with transient or resident organisms from the VN's skin, such as meticillin-resistant Staphylococcus aureus. Gloves are usually latex however sensitive individuals may react to the protein antigen in latex, which may cause allergic dermatitis or systematic anaphylaxis, thus a latex-free option should also be available for sensitive individuals (Gasson, 2010).
Hypothermia
In human medicine perioperative hypothermia is a risk factor for SSIs, as it causes decreased resistance to bacterial infection (Beal et al, 2000). The VN surgical site preparation can contribute to hypothermia particularly when clipping hair and wetting of the skin, consequently VNs must consider hypothermia when preparing their patients for surgery. To reduce the risk of hypothermia, VNs should clip as little hair as necessary, use warm antiseptic solutions and avoiding over wetting of the patient (Lamb, 2009).
Antibiotics
Routine prophylactic antibiotics reduce SSIs, as antibiotics increase the threshold for bacterial infection and reduce, but do not eliminate, the risk arising from bacterial contamination of the surgical wound (Dohmen, 2008). The need for prophylaxis in clean procedures is unclear, and most of the evidence is taken from human medicine, however it is unclear whether human recommendations can or should be directly related to the veterinary field especially as horses live in a contaminated environment.
Clean orthopaedic surgical wounds having an infection rate of less than 5%, thus antimicrobial prophylaxis is generally not necessary, although current guidelines for use in horses suggest that prophylactic antibiotics should be administered, within 1 hour of first incision (British Equine Veterinary Association, 2014), in orthopaedic surgery, this is usually penicillin, which there is no evidence to continue beyond 24 hours after the incision. Additionally there are differences between incision care in equine patients and that of human patients, and infectious complications particularly in racehorses, may be life threatening if they affect long-term performance ability (Wesse, 2008). The horse's environment post operatively is contaminated compared with that of humans. Equine incisions are usually bandaged to either protect the incision from the environment or to minimise movement, thus the incision itself is only checked daily or once every other day, due to the high cost of bandage material; in comparison in human medicine wounds are checked daily or twice daily. VNs can reduce antimicrobial usage by promoting responsible use of antibiotics, and following evidence-based protocols such as using the correct skin disinfectants.
Conclusion
In conclusion, VNs should read peered-reviewed journals, which should be critiqued to facilitate the production of surgical site preparation protocols which include clipping patients, using a good technique, ensuring that well maintained and lubricated size 40 blades are used. Also non-lint gauze swabs should be used rather than cotton wool to prevent microorganisms being transferred onto the surgical field from the lint in cotton wool. In addition, evidence suggests that chlorhexidine gluconate should be used as the skin antiseptic in orthopaedic surgical site preparation. This protocol should ensure gold standard patient care, thus reducing the likelihood of SSIs. However, not all evidence that is published can be implemented in all practices as each veterinary practice's layout varies, therefore each practice needs to have developed their own protocols based on the practice layout. This ensures that all staff follow a standardised protocol for all surgical procedures.
Key Points
Conflict of interest: none.

