Vector-borne infections account for more than 17% of all human infectious diseases globally, causing more than 700 000 deaths every year. They are also a significant cause of disease in cats and dogs, which can act as reservoirs for certain zoonotic vector-borne pathogens. The infection of cats and dogs by UK vectors and their pathogens falls into one of the following five groups:
- Pathogen and its vector already present: fleas and ticks in the UK harbour pathogens of veterinary and human significance such as Bartonella henselae in cat fleas and Borrelia spp. in Ixodes spp. ticks
- Introduction of parasites into existing vector populations ‘vectors waiting for a disease’: Dermacentor reticulatus and Ixodes spp. ticks are already endemic in the UK, and are potential vectors of Babesia canis and tick-borne encephalitis respectively. Mosquitoes capable of transmitting the skin worm, Dirofilaria repens, and heartworm, Dirofilaria immitis, are also present in the UK. These existing vector populations may then become infected through feeding on infected pets, which have travelled abroad or in the case of tick-borne diseases, through infected ticks being introduced on travelled animals
- Introduction of parasites into areas where vectors have spread: new vectors such as Phortica spp. fruit flies have arrived in the UK, which could harbour novel parasites such as the eye worm, Thelazia callipaeda. This parasite is arriving in the UK though travelled and imported pets, presenting the potential for UK fruit flies to become infected
- Introduction of vectors and parasites together: although Rhipicephalus sanguineus ticks are not endemic in the UK, they are frequently seen on travelled dogs. These ticks may be infected with exotic tick-borne pathogens such as Ehrlichia canis potentially exposing the dogs that brought them in and other hosts through household infestation
- Introduction of vector-borne parasites that are then transmitted in the absence of the vector: Leishmania infantum has established in countries such as Canada with no sand fly vector to transmit it purely through venereal and congenital transmission. Cases of leishmaniosis in dogs have also occurred in the absence of the sand fly vector. The mechanism of this transmission is currently unknown.
Educating pet owners regarding potential vectors, pathogens and zoonotic risks along with their prevention, is vital for both pet and human health, as well as wider UK biosecurity.
Determining vector-borne disease risk and preventative strategies
Strategies to limit vector-borne disease rely on a combination of practical control measures and preventative drug treatments; these form the basis of control protocols for individual pets. As the number of treatment options and potential parasite threats increase, parasite control programmes are becoming increasingly popular and important in practice. Parasite control programmes allow bespoke advice to be formulated based on regional risk and the lifestyle of the pet. Asking questions surrounding lifestyle, previous treatment, adverse effects and owner preferences, will also help to maximise compliance, and therefore the effectiveness of any recommended treatment. The collection of information required to formulate a parasite control plan is a team effort and the veterinary nurse (VN) plays a vital role.
Some vectors with disease transmission are ubiquitous and exposure is practically impossible to avoid. For UK cats and dogs this is true of cat fleas. Regular treatment for fleas is essential and should therefore form the basis of all cat and dog parasite control programmes. Prevention against ticks is risk-assessed on the basis of lifestyle and geographical distribution. In addition, imported cats and dogs may be carrying mosquito-borne pathogens, the eye worm T. callipaeda or exotic ticks and tick-borne pathogens. VNs also play an important role in recognising relevant clinical signs in imported pets, which may indicate infection with exotic parasites.
Fleas
Cat fleas are thriving in the UK with wet, warm summers and centrally-heated homes, allowing environmental stages of the flea life cycle to persist the whole year round. This combination of factors leads to increased flea challenge and, without routine preventative treatment, will allow flea infestations to establish (Coles and Dryden, 2014).
Although cat fleas cannot live and reproduce on people, they can bite — leading to human irritation. They are also a source of revulsion, eroding the human–animal bond. They are vectors for a variety of infections including Bartonella spp. (cause of cat-scratch disease), Rickettsia felis (cause of spotted fever) and Haemoplasma spp. (cause of feline infectious anaemia). Flea dirt has been demonstrated to be a significant source of human B. henselae infection via exposure to compromised epidermis without cat-scratch involvement. This is of concern, as a recent study found 11.1% of flea infestations on UK cats and dogs to be positive for Bartonella spp. (Abdullah et al, 2019).
To prevent flea egg laying and break the reproductive cycle, an effective adulticide treatment is required. Adulticides should kill 100% of fleas to prevent egg laying. Adult fleas can lay eggs within 24 hours so effective adulticides must kill fleas within that time period. They must also be administered frequently enough to continue to prevent flea egg laying. The time after application of the adulticide at which fleas have survived long enough to lay eggs is known as the reproductive break point. If the reproductive break point is reached, flea control will fail. Treatment of the environment with insecticides and growth regulators is also important to eliminate existing infestations. Daily vacuuming of areas frequented by flea-infested pets and washing of bedding has also been demonstrated to reduce pupae numbers in the environment. Vacuumed debris should be disposed of after each cleaning session. VN flea clinics provide the ideal opportunity for nurses to discuss the disease risks associated with flea infestations in the home and the measures required for adequate control.
Ticks
The risk of exposure to ticks in the UK has increased, with a milder climate allowing feeding throughout much of the year (Wright et al, 2018). It has been found that 2.37% of ticks on UK dogs, and 1.8% on cats, carry Borrelia spp. and endemic small Babesia spp. such as Babesia vulpes (Abdullah et al, 2016; Davies et al, 2017). In addition, pockets of the tick, D. reticulatus, have been long established in the UK in West Wales, Devon, Essex and London (Medlock et al, 2017). D. reticulatus is the vector for B. canis, a cause of potentially life-threatening anaemia in dogs; and while B. canis had been absent from the UK, these ticks present an opportunity for it to become endemic if introduced through travelling dogs returning from abroad, or through pet imports that may be infected or carrying infected ticks. This risk has become a reality with an endemic foci of B. canis infection establishing in Harlow, Essex, with B. canis being confirmed in local Dermacentor spp. ticks and in untravelled dogs (Phipps et al, 2016). Further untravelled cases were confirmed in Romford in 2016 and Ware in 2017 (Woodmansey, 2017).
Dogs walking in land shared by ruminants and deer, in tall grass, bracken and undergrowth will be at increased risk of exposure. Areas of high tick densities are recognised, however, and have been highlighted in recent UK distribution maps (Abdullah et al, 2016). Dogs living in or visiting Essex should currently be assumed to be at an increased risk of exposure to babesiosis due to the recent outbreak (Phipps et al, 2016). Cats and dogs with a history of tick exposure are likely to be re-exposed. Owners who have reported ticks on clothing or attached to themselves are also likely to have pets with a greater risk of exposure as a result of shared outdoor environments.
No preventative treatment for ticks is 100% effective, but the use of a drug that rapidly kills ticks such as an isoxazoline (Bravecto, MSD Animal Health; Credelio, Elanco; Nexgard, Boehringer Ingelheim; Simparica, Zoetis) or repellents, such as pyrethroids, will greatly reduce disease transmission. With the exception of flumethrin in Seresto collar (Bayer), pyrethroids should never be used on cats due to associated toxicity. However, as no preventative product is guaranteed, pets should also be checked every 24 hours and any ticks found should carefully be removed with a tick hook or fine-pointed tweezers.
Exotic ticks and tick-borne pathogens in imported and travelled dogs
R. sanguineus ticks are capable of carrying a wide range of tick-borne diseases, which are pathogenic to dogs. These include E. canis, Anaplasma platys, Hepatozoon spp. and Babesia vogeli which are frequently being found on travelled and imported dogs. The big tick project, which examined dogs across the UK for ticks, found that of all the dogs that had travelled in the 2 weeks prior to their inclusion in the study, 30.2% were infested with R. sanguineus (Abdullah et al, 2016). ESCCAP UK & Ireland has also seen an increased number of E. canis cases reported in travelled dogs (Stokes and Wright, 2018). Although it is unlikely that R. sanguineus would currently establish outdoor endemic populations in the UK, it can complete its life cycle in 3 months, and larvae nymphs and adults can all feed on a wide range of animals. This allows it to infest UK homes in a similar way to fleas. This is of concern, as these ticks may also carry zoonotic pathogens such as Rickettsia conorii, the cause of Mediterranean spotted fever. Records of R. sanguineus received by the tick surveillance scheme (TSS) are increasing each year, and there have been confirmed house infestations (Hansford et al, 2017). A Hyalomma lusitanicum tick was also recently found on a dog in the UK that had returned from Portugal. This species of tick is a potential vector of Crimean-Congo haemorrhagic fever (CCHF) virus, which is highly pathogenic to people (Hansford et al, 2016). Ixodes spp. ticks, while already endemic across the UK, may be carrying tick-borne-encephalitis, another life-threatening zoonosis. Its presence in imported pets or Ixodes ricinus ticks presents a risk of it establishing in the UK in the endemic Ixodes spp. population.
VNs should be vigilant for common clinical signs associated with imported tick-borne disease:
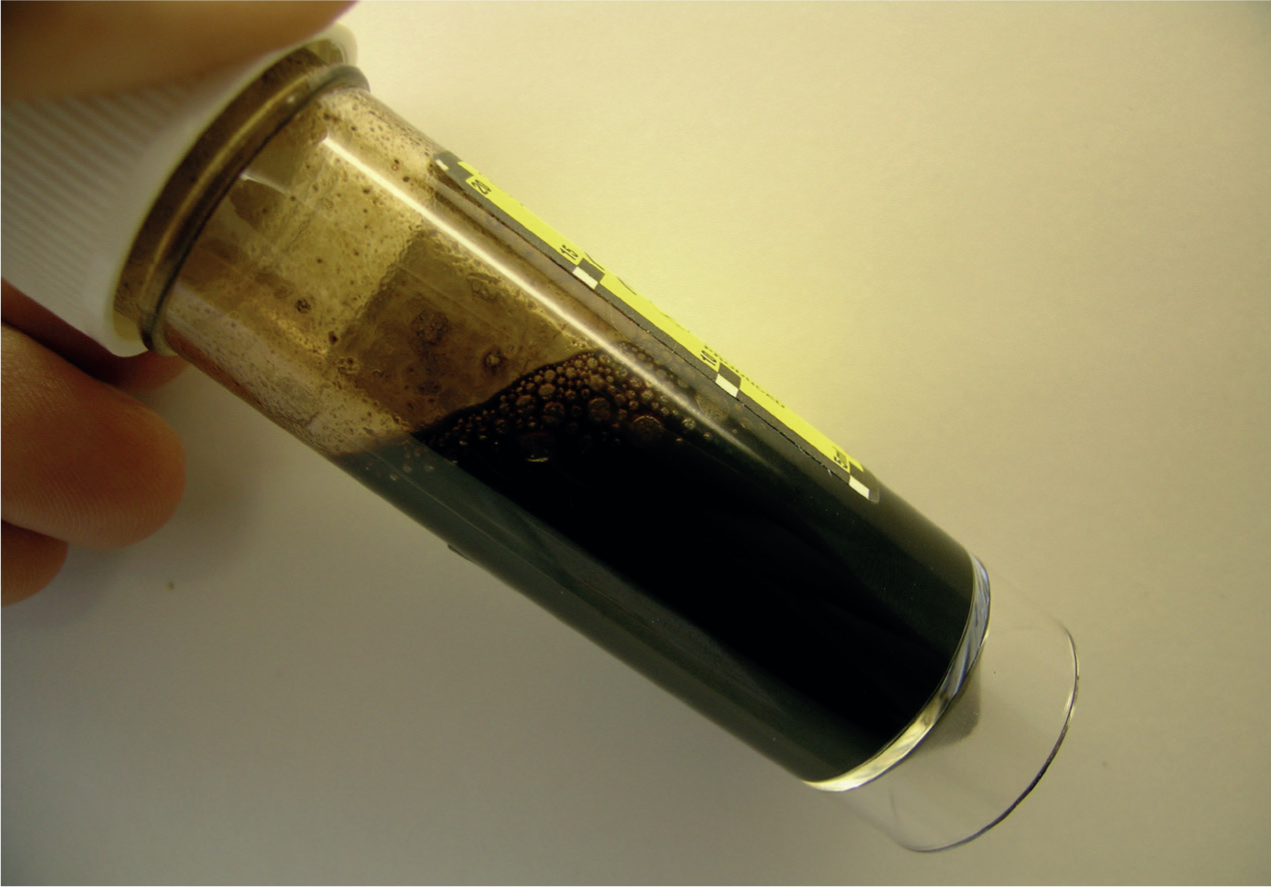
- Anaemia and thrombocytopenia: Babesia spp. infection can lead to immune-mediated haemolytic anaemia in dogs with the development of subsequent regenerative anaemias. Most commonly, these are acute and typically present as pale mucous membranes, icterus, fever and hepatosplenomegaly. Associated depression and anorexia may be present, as well as dark brown urine associated with haemoglobinuria (Figure 1). Concurrent thrombocytopenia may be present with petechiation on the gums. Imported and travelled dogs with these signs should raise suspicion of Babesia spp. infection. Travel history will often be present for acute infections but may have occurred months or years previously, as infection with Babesia spp. is often lifelong and relapses of clinical disease are common. Prevalence of feline Babesia spp. such as Babesia felis is high in South Africa and endemic in other African countries. Cats imported from these countries may be suffering from chronic babesiosis with mild-to-moderate anaemia in the absence of fever. A. platys is a cause of cyclic thrombocytopenia in dogs so this should be considered as a differential diagnosis in travelled dogs suffering from recurrent bouts of thrombocytopenia. It is also a common sign in chronic ehrlichiosis
- Lymphadenopathy and pyrexia: many clinical tick-borne infections transmitted by R. sanguineus will present acutely with lymphadenopathy and pyrexia. Travelled and imported dogs presenting with these signs should be checked for R. sanguineus ticks and house infestation should be considered as a possibility. It is also important to recognise these acute signs of E. canis infection as, without treatment, it may progress to the chronic, often fatal form in dogs
- Neurological signs: tick-borne encephalitis and both acute and chronic ehrlichiosis may present with signs associated with meningitis and meningoencephalitis. These include ataxia, seizures, paresis, hyperaesthesia, cranial nerve deficits and vestibular signs. These may present in dogs with a recent history of travel or, in the case of chronic ehrlichiosis, months or years previously.
Imported and travelled pets should be checked for ticks on arrival to the UK and any found should be sent for identification. Ticks can be identified according to genus using the University of Bristol tick identification site at http://www.bristoluniversitytickid.uk. Alternatively, Public Health England is happy to receive ticks for identification. Ticks should be placed in a container with a secure lid and sent in an envelope marked ‘biological sample’. Samples should ideally be unfed or only partially engorged as features on the scutum and festoons may be obscured in fed ticks. More information can be found on the Gov.UK website at https://www.gov.uk/guidance/tick-surveillance-scheme.
Leishmania infantum in imported cats and dogs
The sand fly vector for Leishmania infantum is not present in the UK so endemic establishment is unlikely (although this has occurred in countries such as Canada with no sand fly vector purely through venereal and congenital transmission). There have also been two recent cases of leishmaniosis in UK dogs with no history of travel. ESCCAP UK & Ireland has seen an increased number of L. infantum cases reported in travelled dogs, with infected dogs regularly seen in practice (Stokes and Wright, 2018).
Leishmaniosis is chronic in nature with a variety of presentations and periods of remission. Signs are due to immune complex deposition in various organs and include:
- Lymphadenopathy
- Cutaneous signs (e.g. generalised and focal alopecia, hyperkeratosis, dermal ulcers and periocular alopecia; Figure 2)
- Weight loss
- Plenomegaly
- Renal signs associated with glomerulonephritis
- Less commonly: polyarthritis, thrombocytopenia, ocular inclusion bodies, uveitis and neurological signs associated with spinal and central nervous system (CNS) granulomas.
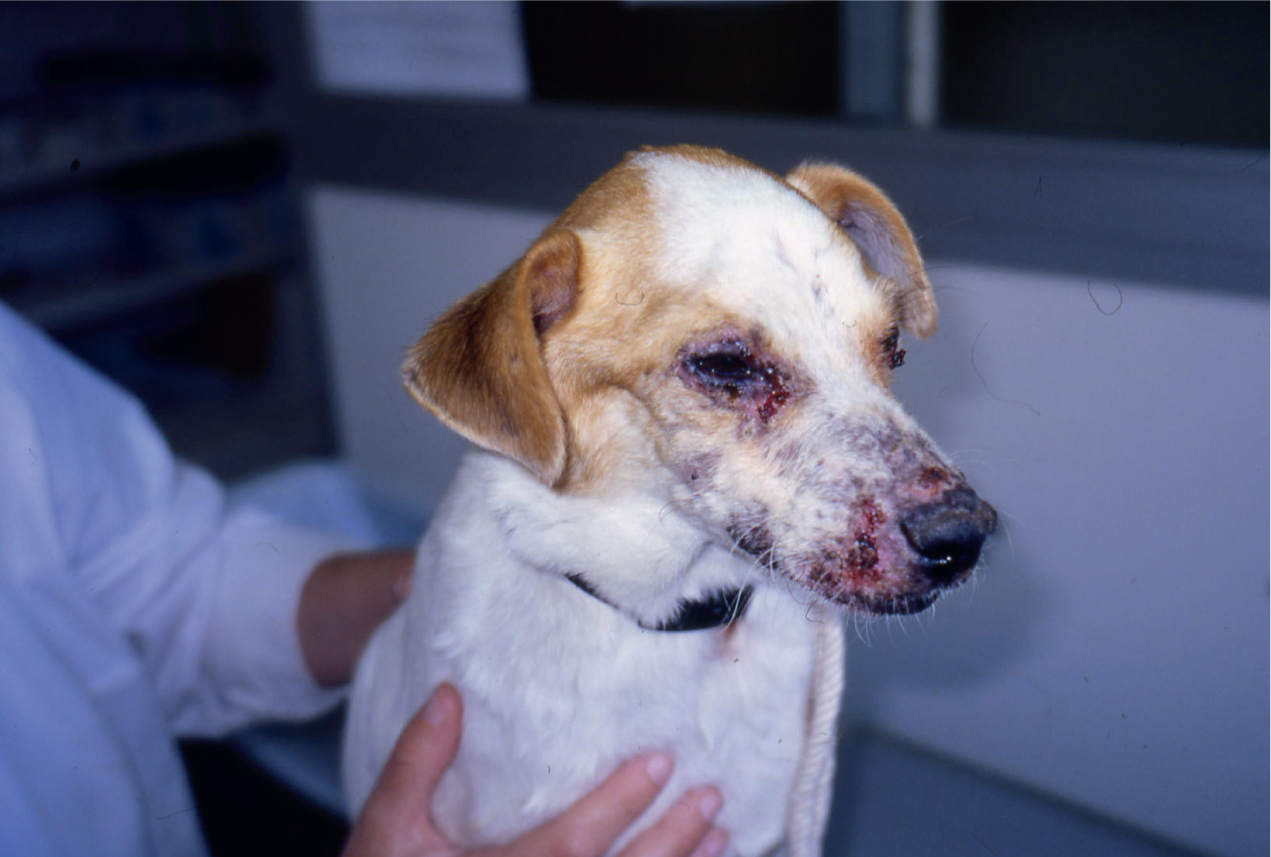
Leishmaniosis should be considered as a differential in imported cats and dogs, as well as those with travel history. Signs may take months or years to develop so foreign travel may not be recent. Infected pets may be subclinical and mixed infections with tick-borne pathogens are common. Therefore, a pet positive for Leishmania spp. infection may have other infections responsible for presenting clinical signs. An association between leishmaniosis and feline leukemia virus (FELV)/feline immunodeficiency virus (FIV) infection has been recognised in cats so these should also be tested for in cats suffering from signs associated with clinical leishmaniosis. Cats may also present with hepatic and gastrointestinal signs.
Thelazia callipaeda in imported dogs
T. callipaeda is a vector-borne eye worm with hosts including dogs, cats and humans. The first confirmed cases in the UK were recently recorded in dogs imported from Romania, Italy and France (Graham-Brown et al, 2017). Should T. callipaeda be introduced to the UK, its primary fruit fly vector, Phortica variegata, has been recorded in the UK with conditions favourable for spread. Although often subclinical, ocular thelaziosis can commonly cause conjunctivitis, keratitis, ephiphora, eyelid oedema, corneal ulceration and, in serious cases, blindness. Close examination of the conjunctiva will often reveal worms actively moving on the surface, and checking is vital in all imported cats and dogs to detect lowgrade or subclinical infections to prevent vector exposure.
Mosquito-borne infections in imported cats and dogs
The first cases of the skin filarial nematode D. repens were recently confirmed in UK dogs imported from Corfu and Romania (Wright, 2017; Agapito et al, 2018). While not highly pathogenic, D. repens is a zoonosis and could be transmitted by mosquitoes endemic in the UK. D. repens infection in dogs appears to be spreading across Europe, with a corresponding increase in zoonotic cases. If infected dogs continue to enter the UK and are not treated quickly, there is the possibility for UK mosquito populations to be exposed to the parasite and for the disease to become endemic.
D. repens infections are most commonly subclinical but clinical signs associated with infection can occur. Dermatitis is the most common clinical presentation as multifocal nodules in the skin or papular dermatitis. These signs can reoccur seasonally for years after infection, resulting in pruritus, erythema, papules and focal or multifocal alopecia. Less commonly, hyperkeratosis, crusting, distinct nodules, acanthosis and secondary pyoderma can occur. Signs may also develop from migration of worms to other parts of the body including conjunctivitis, anorexia, vomiting, fever, lethargy and lymphadenopathy. Ocular migration of worms into the vitreous is uncommon but does occur; therefore, D. repens should be considered as a differential if worms are visualised there and in dogs presenting with dermatitis that have travelled abroad.
Cases in imported and travelled pets of the heartworm D. immitis are also being increasingly reported (Stokes and Wright, 2018). Risk of establishment is also currently low as although mosquitoes capable of transmitting the parasite are present in the UK, the climate has been too cold to allow D. immitis to complete its life cycle. Climate change, however, may allow establishment in the future. Recognition of clinical signs is also important to initiate appropriate treatment in the affected patient. Coughing, tachypnoea, dyspnoea and exercise intolerance are the most common clinical signs seen in infected dogs. Acute clinical signs are associated with thromboembolism, subsequent pulmonary hypertension and caval syndrome. Worm death can also lead to thromboembolism and anaphylaxis. Acute clinical signs may include sudden death, anorexia, weakness, dyspnoea, vomiting and, rarely, respiratory signs linked to pleural effusion. Chronic signs include coughing, dyspnoea, anorexia, vomiting and, less commonly, chylothorax. Chronic respiratory signs tend to be more common in cats.
Education and improving compliance
Planning the consultation
In order to formulate a tailored parasite control plan specific to the client and pet's home-life/routine and geographical location, VNs must develop a working relationship with the client through careful, considered communication, which is both friendly and non-judgmental. By adopting a personalised approach, VNs can create a partnership with their clients in which mutually informed decisions can be made, improving compliance.
There are many opportunities to discuss parasite control in veterinary practice; however, when considering the intricacies of vector-borne diseases, it may be most appropriate to invite clients to attend parasite or travel nurse clinics. Alternatively, a client awareness evening may provide the opportunity to educate a larger number of clients on vectorborne diseases; this would then need to be followed with a consultation to create the individual parasite control plan.
The VN who conducts these clinics should be knowledgeable and passionate about the importance of tailored parasite control plans. If the VN is also a suitably qualified person (SQP), this is beneficial as it enables the VN to prescribe and supply some anthelmintics under the remit of their qualification, e.g. a C-SQP can prescribe for companion animals. This, however, is not essential and it is often useful to run these clinics in conjunction with a veterinary surgeon (VS). If more than one VN is going to be involved within the clinics, it is vital that the information given is standardised; while we want to tailor the advice given to the specific needs of the client and pet, we also want to make sure the general principles discussed are the same. Completing training sessions and having meetings with all staff involved in the clinics can be useful to ensure everyone adopts the same approach. Continuing professional development (CPD) should also be undertaken and the authors recommend that veterinary nurses regularly consult the European Scientific Counsel for Companion Animal Parasites website (https://www.esccap.org/vectors), as vector-borne parasites and their associated diseases are complex, diverse and ever-changing so remaining up to date is essential.
When beginning the consultation, VNs should ensure the room used is clean and presentable. Consideration should also be given to the layout of the room. For instance, barriers to communication such as the table blocking the room, or one person sitting while the other stands can hinder effective communication and these factors should be minimised. During a busy working day, consultations may be rushed due to time constraints; however, it is vital that the VN takes a few moments to familiarise themselves with the pet's clinical history (if available) and to plan the consultation.
Use of a structured framework to guide effective communication
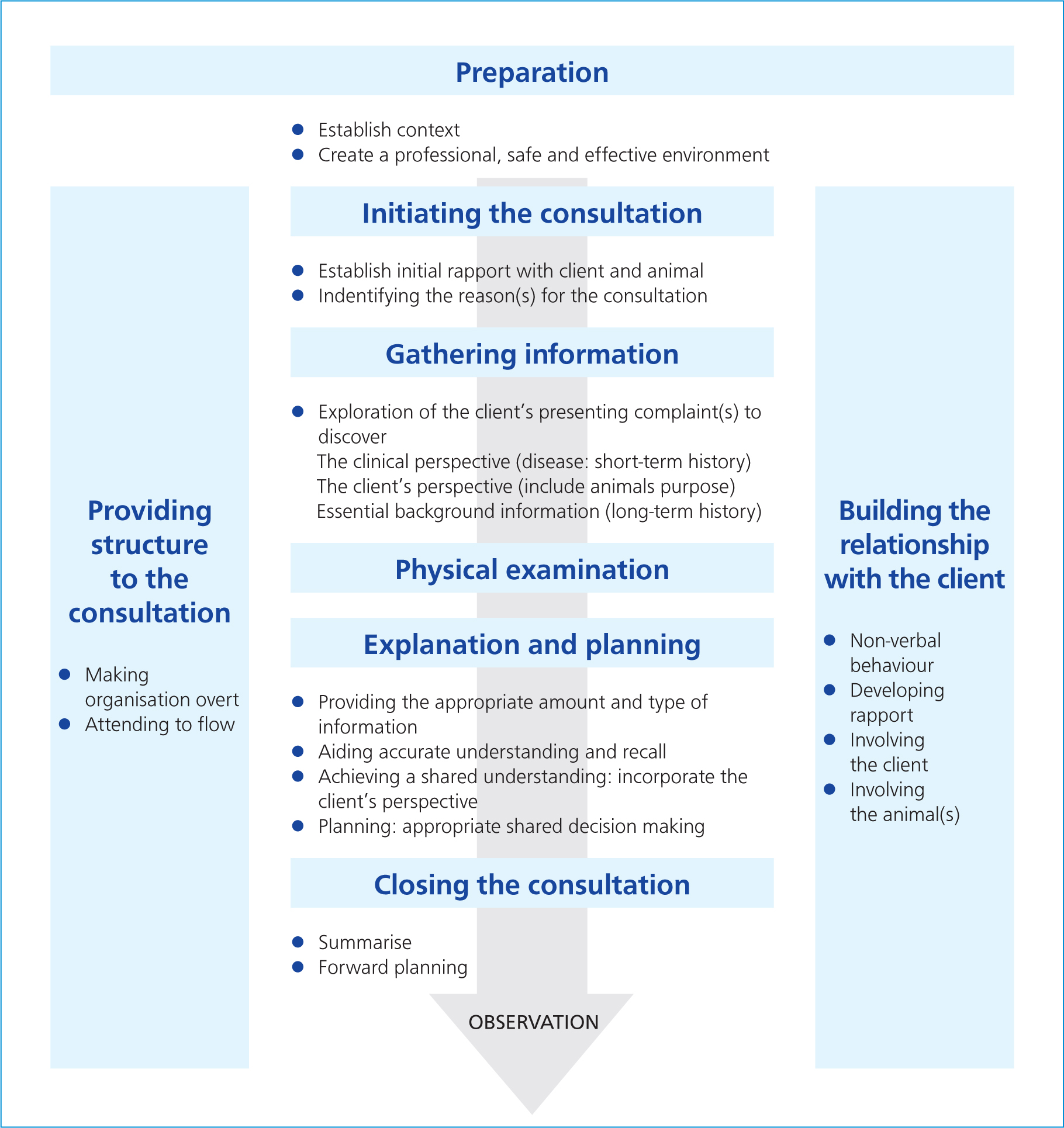
The Cambridge-Calgary consultation model (Figure 3) was adapted by the National Unit for Advancement of Veterinary Communication Skills (NUVACS) and is therefore suitable for use by veterinary professionals. The model provides VNs with a structure/framework on how to gather information, explore it further and then summarise the information to the client (Ackerman, 2012; Wiggins, 2016). The model also encourages the user to implement further communication techniques to enhance the conversation, such as active listening.
Active listening is a technique which enables both the VN and client to ensure their opinions have been heard and understood correctly. When the VN asks a question, the client's answer should be repeated or form part of the next question; this provides the client with an opportunity to expand on their answer or confirm the information is correct. VNs must balance the use of both open and closed questions. During an assessment of parasitic risk, there are certain elements of information the VN must obtain. This can be achieved via the use of closed questions, e.g. when was the pet last treated? By also using open questions, the client is given the opportunity to offer information and explain their opinions/views. This mixed style of questioning is vital and helps to strengthen the partnership between client and VN; clients who feel their opinions have been heard are more likely to act on the advice given (Ackerman, 2012; Loftus, 2012).
In addition to consulting models, there are also 7 Cs of communication (Table 1); consideration of these factors can help aid effective communication (Hedberg, 2016).
Table 1. 7 Cs of communication
| The C | Information |
|---|---|
| Complex | Keep it simple, consider the talking speedLanguages and accents can act as a barrier to communication |
| Content | Plan what will be discussed (a consultation model may be helpful as discussed), consider the pitching level of the information; simple is often best |
| Context | Keep it relevant. Sometimes a consultation may go off course; this is fine but ensure the primary aim has been met |
| Concentrate | Make sure the room is quiet and free from distractionsListen closely to the owner |
| Courtesy | Maintain a pleasant facial expression and open body positionRespect the owner's opinion |
| Consistency | Standardise advice given by all staff, but ensure a personal approach is still adopted |
| Compassion | Demonstrate empathy for the owner's opinions and views |
The 7Cs highlight the importance of not only verbal communication, but also the non-verbal cues that may be overlooked such as body language and facial expression. All of these factors play a vital role in creating a successful partnership with clients and therefore improving compliance.
During conversations with clients, the VN must ensure the information is pitched at the correct level and that technical/medical terms are avoided to ensure the information is portrayed clearly. Thought should also be given to the client's learning style by using a variety of teaching methods, e.g. pictures, videos, diagrams, demonstrations and practical sessions. By using different methods, the VN can cater for all styles as well as make the consultation interactive.
Assessing parasitic risk
When assessing parasitic risk, VNs must ask the client a series of questions regarding the home life and geographical location of the client and pet. When considering the potential for vector-borne diseases and parasites, we must also ask the client about the pet's background (see questions below), this is of particular importance if the client has not owned the puppy/kitten from a young age as there may have been exposure to vector-borne diseases/parasites that the client is unaware of.
There are several general questions that must be asked when formulating a parasite control plan. They are important as they allow the VN and VS to tailor the control plan to the individual client and pet, which results in improved compliance. The following list is not exhaustive:
- What parasite control treatment does the client usually use? When was the pet last treated? What is the frequency of administration? What was the product used? By asking about the normal parasite control plan of the client and pet, the VN can determine when the pet can be next treated and what active ingredient may be appropriate. Drug interactions can occur if the active ingredient is the same or from the same group. The VN should consult the VS regarding this, as knowledge of drug groups is essential. By questioning about the frequency of administration, the VN can advise if this is appropriate (e.g. is it effective to prevent egg laying in fleas, is the reproductive break point being reached?)
- Does the client regularly treat the house for fleas? Use of an insect growth regulator is vital to reduce the environmental stages of fleas and therefore break the life cycle. The VN must also discuss daily vacuuming (the vacuum cleaner should be emptied after each use) in all areas frequented by the animal, in addition to washing bedding and soft furnishings at 60°C
- Has the pet had any previous reactions to treatment? Or does the client perceive the pet to have had a reaction? Some animals may vomit following anthelmintic administration; others may hypersalivate following application of a spot-on treatment. By avoiding drugs in which a reaction has occurred, risk to the patient is minimised and compliance increased
- Does the client prefer a specific product formulation? Are they confident in administering tablets, spot-ons, etc? To ensure the client complies with the advice given and administers the products as recommended, it is vital to choose a product that fits the needs of the pet, but also that the client feels happy to administer. If the client has no experience, training can be provided
- Does the pet go swimming or is he/she regularly bathed? Continued use of products, which rely on being absorbed into the sebum layer (e.g. fipronil and imidacloprid) with concurrent regular bathing and/or swimming, can result in poor compliance due to a perceived lack of efficacy. In these cases, it would be more appropriate to choose a different product which does not work in this way
- Does the client have children or grandchildren? A monthly parasite plan should be advised in these cases to limit the risk to the children of human toxocarosis
- Does the client have other pets? All pets within the household should be treated; animals which do not go outside can still be infected via parasites brought in by other animals or the client.
In addition to these general questions, below are a series of questions which should be considered when discussing vector-borne parasites and associated diseases (not an exhaustive list):
- Is the client aware of what vectors are? Is the client aware of the transmission for parasites and diseases via vectors? Use of open questions helps the VN to gauge the client's knowledge level on vector-borne diseases and parasites. The VN can then discuss this more generally before moving on to specific parasites which may pose a risk to the pet
- Has the pet travelled abroad? The VN should gain a full travel history including recent and historic travel. Any history of travel could prompt the potential for vector-borne diseases. Not all present acutely; some can develop clinical signs over time or have waxing and waning symptoms
- Has the pet been imported? With the rise in imported dogs and cats, it is vital this is also taken into consideration
- Does the pet regularly walk in land shared by ruminants or deer, or in tall grass or bracken? Does the pet live in or frequently visit areas of high tick densities? Does the pet have a history of tick exposure? If the answer is yes to any of these questions, the pet is considered to be at increased risk of exposure to ticks and therefore tick-borne pathogens and diseases such as babesiosis. The client should be advised to regularly check both themselves and their pet for ticks and remove using a tick hook in addition to regular tick prophylaxis.
Recognition of relevant clinical signs
Diagnosis is an act of veterinary medicine as detailed in the Veterinary Surgeons Act 1966 and can only be performed by a VS. Despite this, VNs can play an active role in the diagnostic process by gathering information for the VS.
The clients' answers to the questions outlined in the previous section may prompt further discussion in certain areas. While the aim is to discuss and formulate a parasite control plan, sometimes consultations may go off on a tangent to deal with any concurrent issues. As discussed, the clinical signs associated with vector-borne parasitic diseases are often vague; however, these signs coupled with the history of the client having removed a tick or the pet having travelled may trigger a warning for the VN. At this stage, however, it is vital not to scare the client as the two factors could be unrelated. If there is any suspicion or the animal is unwell, the VN should consult a VS for further discussion and/or diagnostics.
General clinical signs the VN should be mindful of include (not an exhaustive list):
- Lymphadenopathy
- Vomiting
- Pyrexia
- Lethargy
- Depression
- Weight loss
- Polyarthritis
- Seizures
- Ataxia
- Dermatitis
- Conjunctivitis
- Renal, hepatic or gastrointestinal signs.
As demonstrated, the signs are non-specific and there is crossover between the diseases and parasites mentioned. In addition, it is possible for mixed infections to be present.
Conclusions
Vector-borne diseases are complex and there is potential for the spread of new parasites and diseases within the UK due to changes in climate and pet travel guidelines. It is vital that the VN has an awareness of the various risks these present to both the pet and client, and the signs which could indicate potential infection. The VN must work in consultation with the VS and the rest of the veterinary team to create tailored control plans for the client and pet.
KEY POINTS
- Vector-borne infections affect both humans and animals. The changes in climate and pet travel guidelines mean there is potential for the introduction of new vectors or parasites which present a risk to health.
- The veterinary nurse plays a vital role in educating clients on the risks of vectorborne parasites and their associated diseases.
- Compliance can be increased by communicating effectively with clients to improve knowledge and, subsequently, the health and welfare of the pet.
- Through careful questioning, veterinary nurses can determine individual risk and create a partnership with clients in which tailored plans are created based on informed decisions.


