Preventing infection in critically ill patients is a very important daily activity for which there is very little research to guide best practice for veterinary patients, and fairly limited information for human healthcare practictioners (National Institute for Health and Care Excellence, 2012). In the author's experience, veterinary infection control practices tend to be more diligently followed in situations where zoonotic diseases are under consideration, e.g. leptospirosis patients. In addition, such highly contagious animal diseases also tend to heighten awareness and prompt effective implementation of infection control practices. Unfortunately, critically ill veterinary patients are at high risk for developing many nosocomial infections that might not otherwise be considered highly contagious (Kirby, 2009). Human behaviour in situations where there is not a perceived risk tends towards non adherence to many effective infection control practices. Human and veterinary infection control practices have gained heightened attention recently due to the emergence of meticillin-resistant Staphyloccocus aureus (MRSA) and meticillin-resistant Staphyloccocus pseudintermedius (MRSP) in addition to several other multi-drug resistant organisms in human and small animal critical care practices around the world (National Institute for Health and Care Excellence, 2012; Weese, 2012). Research on this topic has shown many times that effectively implemented guidelines rely on common sense and knowledge of basic principles, they must be suited to the local environmental conditions and problems, and healthcare workers must understand the principles and be effectively supported in order to achieve successful change. Simply making a policy and expecting it to be enforced is unlikely to accomplish significant change. Infection control practices can be broken down into several important areas: hand hygiene, cleaning and disinfection of facilities and equipment, appropriate use of isolation facilities, and antibiotic use stewardship (CCAR-CCRA, 2008).
In the author's opinion, all patients presented to veterinary clinics should be evaluated to assess their infectious disease risk. This should be done by the primary veterinary surgeon in charge of the case but may be delegated to nursing staff. Staff, including reception staff, should be notified in regard to outpatients with signalment, history, physical findings, and/or laboratory results that is suggestive of contagious disease, so that appropriate cleaning and disinfection procedures may be instituted. Patients being admitted into veterinary clinics (day patients, overnight or long term) should ideally be assigned a biosecurity status, again based on signalment, history, physical findings and/or laboratory diagnostics. This should be done as soon as possible, preferably before the patient leaves the examination room or treatment area. This will determine where the patient will be hospitalised and the order of care. The appropriate biosecurity status should be placed on the cage hospitalisation sheets (see below). The responsibility for assignment of appropriate biosecurity status ultimately rests with the veterinary surgeon in charge of the case. It is also the responsibility of the veterinary surgeon to ensure that prescribed biosecurity standard operating procedures (SOPs) are instituted and followed. This responsibility may be delegated to a qualified staff member, e.g. a senior nurse. Deliberate deviations from protocol are discouraged but permissible when considered to be in the best interest of the patient or the safety of personnel. Justification for such deviations should be briefly noted in the patient record and initialled by the veterinary surgeon in charge of the case.
Disease transmission
Disease transmission is one aspect of an infection control strategy or protocol, which should be considered for every patient.
Three elements are required for successful disease transmission:
Source of infection — animal sources of infection can include endogenous microflora that are pathogenic to humans. Environmental sources of infection can include contaminated walls, floors, worktops, cages, bedding, equipment, supplies, feed, soil and water.
Host susceptibility — host susceptibility to infection can vary greatly among the general population, with increased susceptibility seen in the unvaccinated, the very young and the elderly, those who are immunosuppressed, pregnant or those with injuries that would allow a break in the normal defense mechanisms. These patients should also be considered when designing infection control protocols as these patients will be highly susceptible to infections.
Routes of transmission — pathogens are transmitted through three main routes: direct contact, aerosol and vector-borne transmission (Elchos, 2008).
Direct contact can occur through ingestion of the pathogen, puncture wounds such as needlesticks or bites or mucous membrane exposure (CCAR-CCRA, 2008). Indirect transmission can occur through exposure to fomites as when cleaning cages and equipment, or through handling dirty laundry.
Aerosol transmission occurs whenever a pathogen travels through the air. This can be through large droplets that are deposited on the mucous membranes or through smaller particles that can be inhaled. In general, the risk of aerosol transmission increases with proximity to the source and duration of exposure (Elchos, 2008).
Large droplets can be generated by coughing, sneezing and vocalisation (CCAR-CCRA, 2008). An individual can also be exposed through procedures such as lancing abscesses and dental procedures. Particles may also be aerosolised through the use of suction units, bronchoscopy, and sweeping, vacuuming and high-pressure spray washers (CCAR-CCRA, 2008). Once aerosolised, certain pathogens may remain infective over long distances. This is dependent on particle size, the nature of the pathogen and environmental factors such as temperature and humidity (Elchos, 2008).
Vector-borne transmission occurs when vectors such as fleas and ticks are transmitting the disease during their normal feeding activities (CCAR-CCRA, 2008). Working in outdoor settings may also increase the risk of exposure to insects and other biological vectors (Elchos, 2008).
These routes for disease transmission need to be taken into consideration whenever hospitalising patients, and determining their biosecurity status.
Infection control strategy
Example of infection control strategy (biosecurity) in the author's practice.
Tier 3 patients may be hospitalised in the general ward areas, provided appropriate attire is worn. If intensive care is required, cages in the ICU may be used. In ICU, a perimeter around tier 3 cases should be clearly marked on the floor when the possibility of contamination with urine or faeces exits, e.g. leptospirosis, giardiasis, campylobacteriosis. When using ICU, consideration should be given to the risk posed to other patients in the ward, especially tier 1 cases. Patients infected with MRSA should only be hospitalised in isolation.
Tier 4 patients should be placed directly into small animal isolation without transiting through ward areas. If the patient cannot be carried, a gurney should be used for transfer. Examination rooms should be clearly marked as contaminated and the veterinary staff and front desk notified so that appropriate cleaning and disinfection measures may be undertaken. Tier 4 patients must remain in isolation until discharged.
Additional considerations also need to be paid to specific areas of the hospital, e.g. room type, furniture etc. Hygiene protocols should be designed for each of these areas so staff know when and how that environment, and the equipment within it, should be cleaned and disinfected, and who is responsible. These protocols should be tailored to the specific veterinary clinic, but should include protocols for all areas, e.g. waiting room, consulting rooms, laboratory, wards, theatres, prep areas etc covering ceilings, walls, floors, doors, handles, switches, windows, blinds, computers, furniture, e.g. desks, tables, trolleys, machines etc (CCAR-CCRA, 2008).
Hand hygiene
Hand hygiene is one of the most important aspects of infection control within hospital environments, but is one of the most difficult to enforce. Most individuals when asked indicate that their own hand hygiene practices are acceptable and regularly performed. In fact, adherence to hospital guidelines in all appropriate situations is rarely as high as would be expected (Nakamura, 2012). There are numerous reasons that have been cited as contributing factors for non-adherence including: guidelines too complex and hard to follow, too busy, lack of education about importance, hard to change old habits, and good hand hygiene practices cause drying and dermatitis (Nakamura, 2012). Hand hygiene implementation plans have to take all of these factors that may affect adherence into consideration when guidelines are developed and deployed.
Adherence to hand hygiene practices can be improved through a number of methods. It is important to include staff in product selection and choices. Personal preference and individual reaction to different products may assist in adherence due to fragrance, consistency, or interactions with skin during regular use. Human behaviour and motivation plays a significant factor in successful implementation of hand hygiene policy (Kampf, 2009; Nakamura, 2012). Education is extremely important. Healthcare workers must understand the importance of hand hygiene, the ramifications of poor practice, and correct techniques. Clearly defined and objective outcomes that are measured and reported frequently can also serve to both motivate and remind veterinary staff and promote adherence (CCAR-CCRA, 2008).
Cleaning and disinfection
A second front of infection control practice is cleaning and disinfection. With the advent of MRSA and MRSP in veterinary hospitals, cleaning and disinfection have also taken on heightened importance. S. aureus is a very hardy organism in the environment and can be quite resistant to desiccation in a compatible environment. Recovery of organisms from the environment has been documented months and years after the environment becoming infected (Dancer, 2008). A dirty environment or one with a significant biofilm allows protection of the organism and facilitates the spread of infection (Dancer, 2008). Thus cleaning and disinfecting has taken on heightened significance. Of most importance in spread of this epidemic are routine cleaning of contact surfaces such as door handles, light switches, computer keyboards (Figure 1) or mice, and the undersurface of tables and other commonly gripped or touched areas that are not part of a routine cleaning regimen. These commonly touched but less routinely cleaned surfaces serve as a major source of the spread of zoonotic organisms such as MRSA from patients to humans and back again and must be addressed effectively in the face of any outbreak (CCAR-CCRA, 2008).

Although cleaning and disinfection would at first glance seem to be easily understood, there are many important principles to be considered. Effective cleaning and disinfection protocols address as many microorganisms as possible, and take into consideration product compatibilities and synergies while minimising any risk of incompatibility or patient risk from toxic substances. Most importantly, the local ‘problem’ microorganisms must be considered carefully and products chosen to deal effectively with them (CCAR-CCRA, 2008).
The first step in any cleaning protocol is to use a detergent that effectively cleans the biologic material and breaks up the biofilm from the surface. Application of the detergent with vigorous scrubbing and hot water also facilitates the goals of this step in cleaning. Next the detergent must be effectively rinsed from the surface, leaving no residue, as many disinfectants can be neutralised by the presence of residual detergents. Residual standing pools of water can dilute the disinfectant that is applied next (CCAR-CCRA, 2008). In addition, they may serve as reservoirs for replication and survival of microorganisms. Therefore, standing pools of water should be drained or removed prior to application of disinfectant. Last, a disinfectant solution should be applied at the correct concentration and within the correct period of time following preparation to maximise the potential to be effective. In addition, most disinfectants must have sufficient contact time to effectively kill many microorganisms. Thus, the disinfectant solution in many circumstances should be left to dry on the surface to allow maximal killing time or effectiveness (Wortinger, 2011a).
There are many issues to consider when choosing a detergent and disinfectant product that are unique to each specific situation, and are best covered by reviews of these products (Hall, 2010) Of significance however, is the study of resistance genes to disinfectants. Many bacteria appear able to also develop resistance to specific disinfectant classes, in a similar way to antibiotic resistance. Thus, in specific outbreak situations, it may be important to consider this as one possibility of how an otherwise effective approach to cleaning and disinfection has failed and contributed to the outbreak (Wortinger, 2011a). The author would recommend consultation with experts in the field of cleaning and disinfection when dealing with any specific outbreak, or local environmental problems. Hospitals should consider routine surveillance of the environment as an important component of an effective infection control practice (CCAR-CCRA, 2008).
Isolation facilities
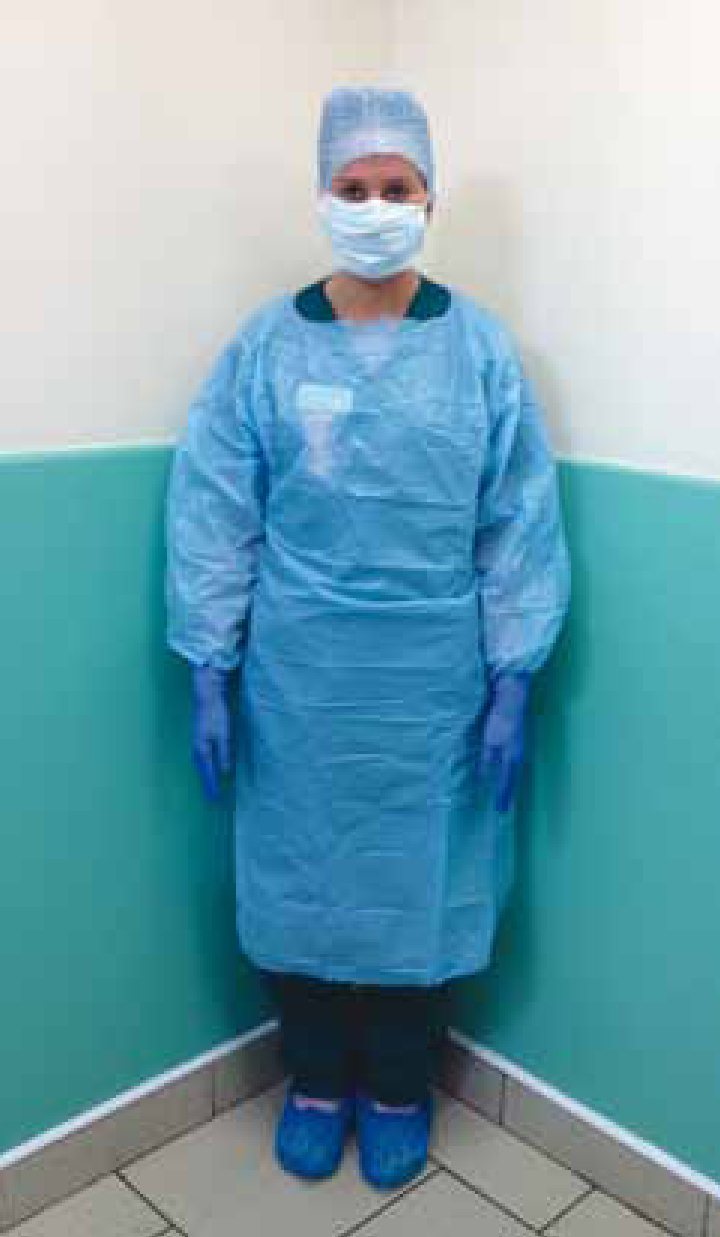
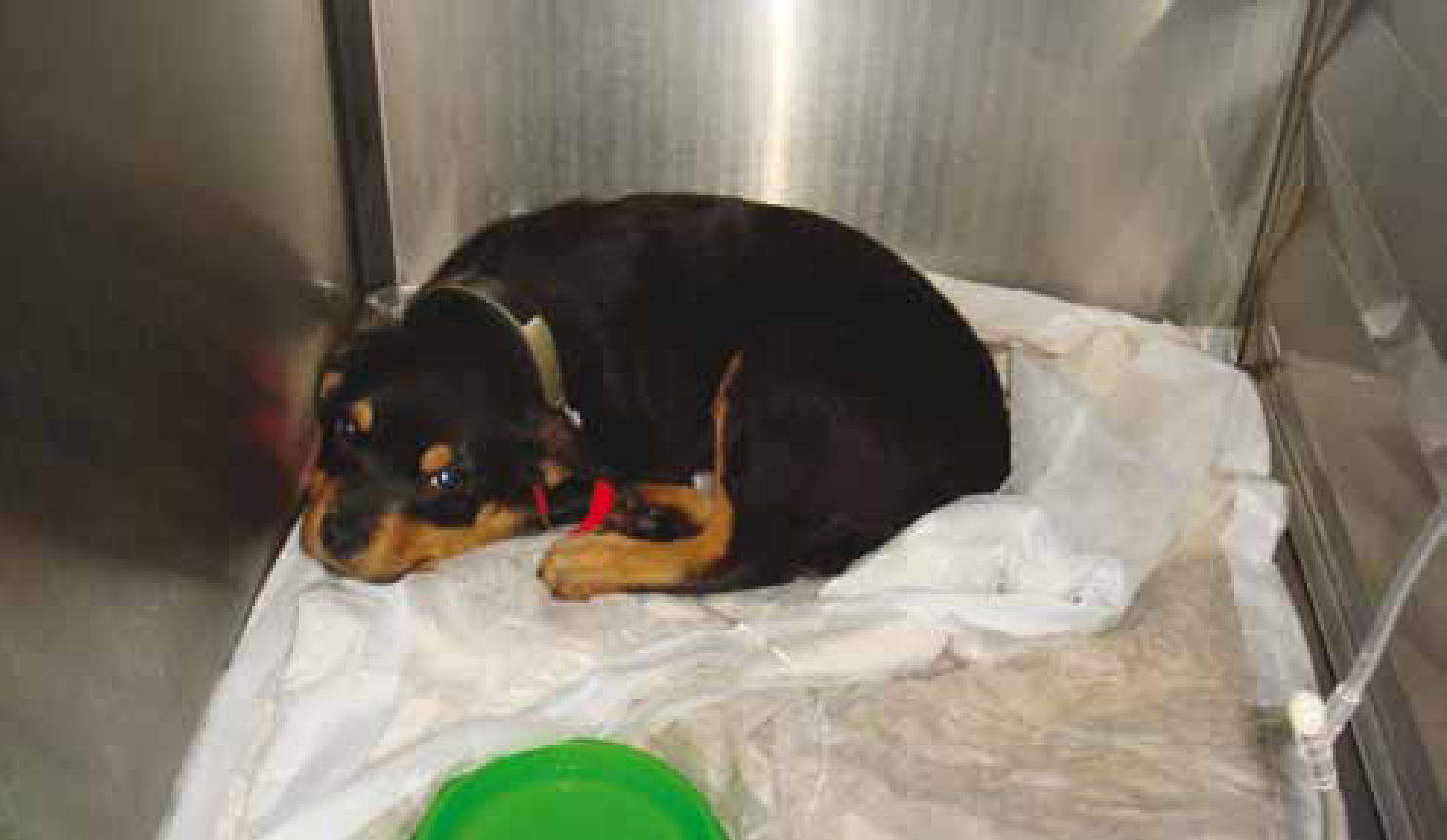
A third important aspect of infection control practice is the isolation of patients that serve as an important risk to either humans or other patients. In most cases, isolation facilities in veterinary hospitals have been poorly designed and have often been an afterthought during construction (CCAR-CCRA, 2008). Because they are infrequently used in many circumstances, practice owners or hospital administrators are reluctant to invest large amounts of resources into proper construction. Most importantly, isolation facilities must allow isolation of individuals from one another; a concept that is very rare in small animal veterinary facilities. To be effective, isolation spaces must also have an area where individuals entering the isolation space can put on and remove appropriate and effective personal protective equipment or devices to protect themselves and prevent the spread of infection (CCAR-CCRA, 2008). Such devices, such as wearing a gown, can be perceived by healthcare workers as an impediment or a hassle and therefore, education and monitoring of adherence are extremely important facets of an effective policy (Wortinger, 2011b). Healthcare workers should also be educated or have guidelines to consult regarding what personal protective equipment is necessary (Figure 2) depending on the known or suspected pathogens in question (CCAR-CCRA, 2008). In the author's opinion these concepts are even more important in veterinary emergency and critical care since many patients with severe infectious disease (e.g. parvovirus diarrhoea) (Figure 3) require continuous care, which means that emergency/critical care staff, by nature of their 24 hour responsibilities are required to care for such patients and then step back into the emergency or critical care facility and care for other patients that may be at high risk for developing infectious diseases. Thus, effective use of isolation facilities and ensuring adherence to established guidelines and protocols is critical for veterinary facilities to prevent nosocomial spread of disease.
Key Points
Antimicrobial stewardship
Finally, it has become increasingly important for veterinarians to be responsible stewards of the antimicrobial drugs they utilise. As more is discovered about antibiotic resistance, the use of antimicrobials in companion animal practice and not just food production circumstances will continue to come under more and perhaps harsher scrutiny. The documented ability of certain microorganisms to share resistance genes places all antibiotic use under the microscope (Fishman, 2006). The mechanisms of antibiotic resistance are numerous.
Conclusion
Designing an effective infection control strategy is multifactorial and should be designed with a specific clinic in mind. Staff training is an additional factor, to ensure all new staff are aware of the practice protocols, and how they should be carried out, and also to ensure existing staff are up to date, and their knowledge is refreshed on a regular, on-going basis.
