The use of cuffed endotracheal (ET) tubes in cats is a controversial subject. For many years there has been reluctance by some veterinary surgeons to use cuffed tubes, while others use them on a daily basis without considering them cause for concern. The aim of this article is to examine current literature and research, from both the veterinary and human field, with the aim of highlighting causative factors that may contribute to tracheal damage as a result of intubation using cuffed ET tubes. The article will also suggest good practical techniques that can be employed to reduce tracheal damage if cuffed intubation is utilized in practice.
Tracheal injury as a result of cuffed intubation in the UK
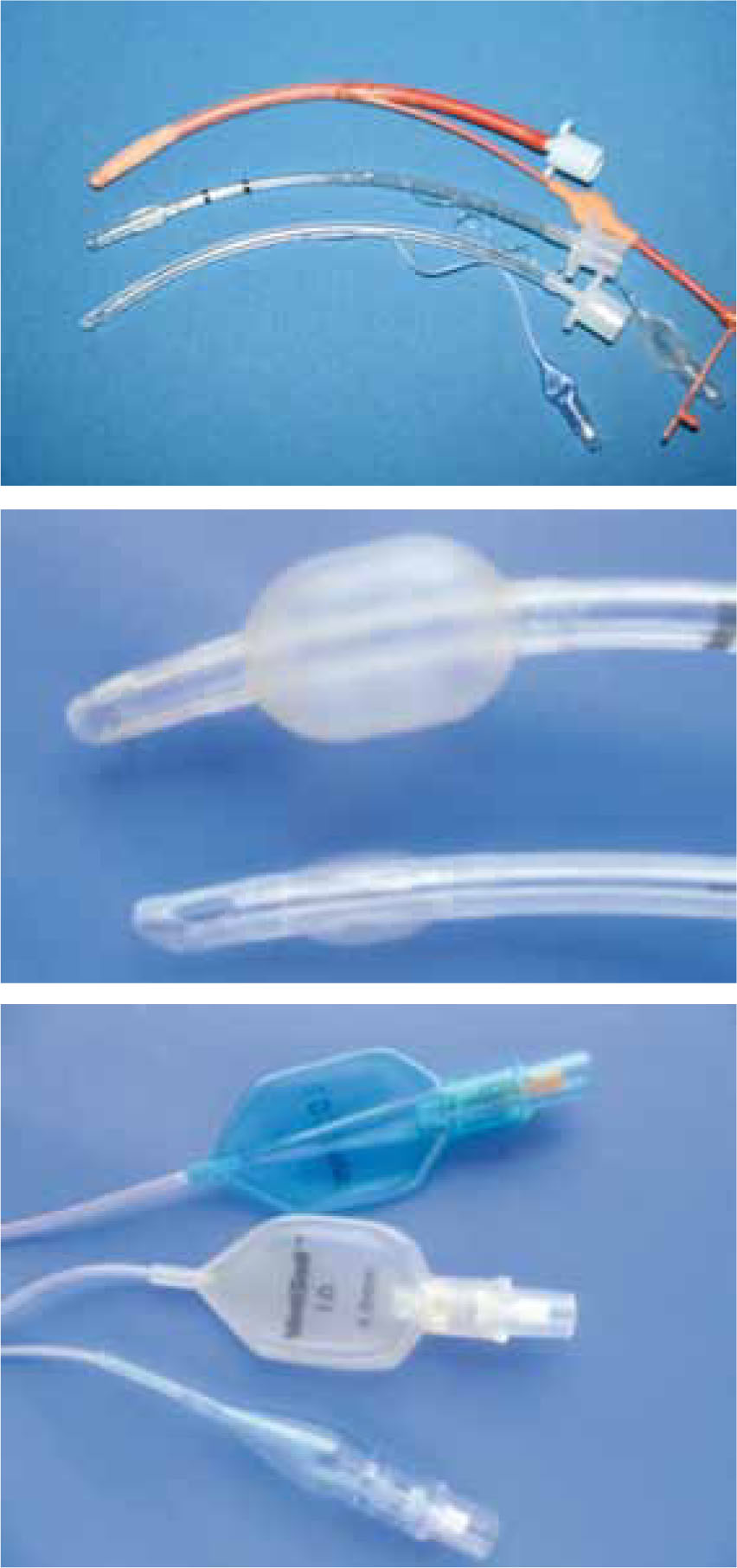
Over the past 10 years the Veterinary Defence Society (VDS), a mutual insurance company that insures the practising profession against claims of negligence, reported 11 feline claims involving ruptured tracheas as a direct result of cuffed ET tube intubation (Hird, 2010). The 11 claims amount to nearly 1% of all VDS feline claims, and although small, the significance increases when you consider that not all veterinary practices are insured by the VDS and not all tracheal injuries result in a VDS claim. It is therefore fair to suggest a higher number of feline patients may have been exposed to tracheal damage related to intubation with cuffed ET tubes. Interestingly, all of the 11 claims reported were in cats anaesthetized for dental treatment and all 11 cases were intubated with red rubber high pressure, low volume (HPLV) cuffed ET tubes (Figure 1).
Anatomical considerations
The cat's trachea is a cartilaginous and membranous tube that serves as an airway between the larynx and the bronchi. It consists of a series of incomplete rings of hyaline cartilage joined by connective tissue and the trachealis muscle that runs along the complete length of the trachea. These incomplete rings and the trachealis muscle are beneficial because they give the trachea a great deal of fexibility and elasticity. The only problem is that this is the area that is damaged during excessive cuff inflation, resulting in a longitudinal tear down the join between the trachealis muscle and the tracheal rings (Kastner et al, 2004). The dog's trachea is anatomically very similar and, like the cat's, is formed with incomplete cartilaginous rings joined by connective tissue and the trachealis muscle. The only difference is that tracheal injury related to cuffed ET intubation appears to be less common in the dog and the VDS, in the last 10 years, have had no reported cases of tracheal injury related to cuffed intubation in the dog (Hird, 2010). This is not to suggest that tracheal injury will not occur in the dog but does highlight that cats are at higher risk of tracheal injury, presumably because the trachea is generally much smaller and more delicate compared with the dog's, leaving a smaller margin for error during cuff inflation.
Another consideration relating to the anatomy of trachea is the perfusion pressure of the tracheal mucosa. To ensure adequate perfusion and oxygen delivery to the cells of the trachea the perfusion pressure within the capillary beds has to be maintained between 25–35 mmHg (Hartsfield, 2007). If the cuff exerts a mucosal contact pressure greater than the capillary perfusion pressure then obstruction of the capillary blood flow can occur, which can result in ischaemic injury of the tracheal mucosa. To avoid ischaemic injury it is recommended that the lateral wall pressure exerted by the cuff should be maintained at less than 18–24 mmHg and should never exceed the upper limits of the capillary perfusion pressure noted above (Hartsfeld, 2007). Slatter (2003) even identifies a cuff inflation pressure of 15 mmHg as being suficient to provide a good seal between the ET tube and tracheal wall (Figure 2).
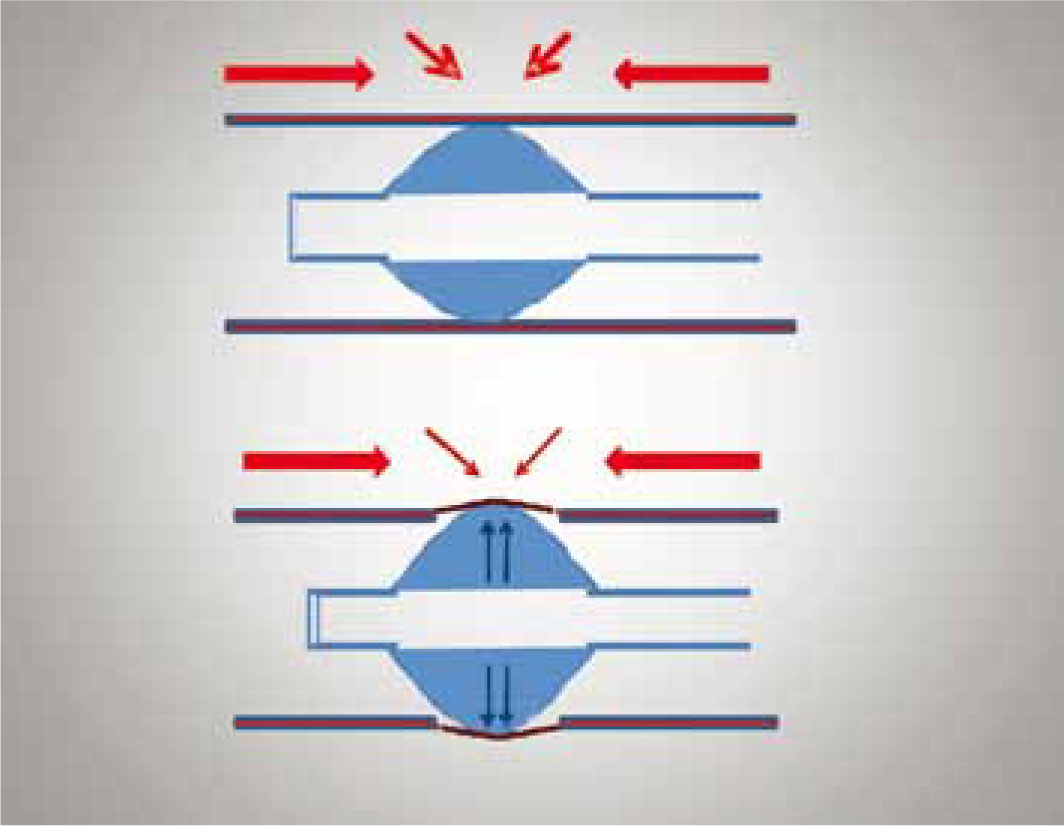
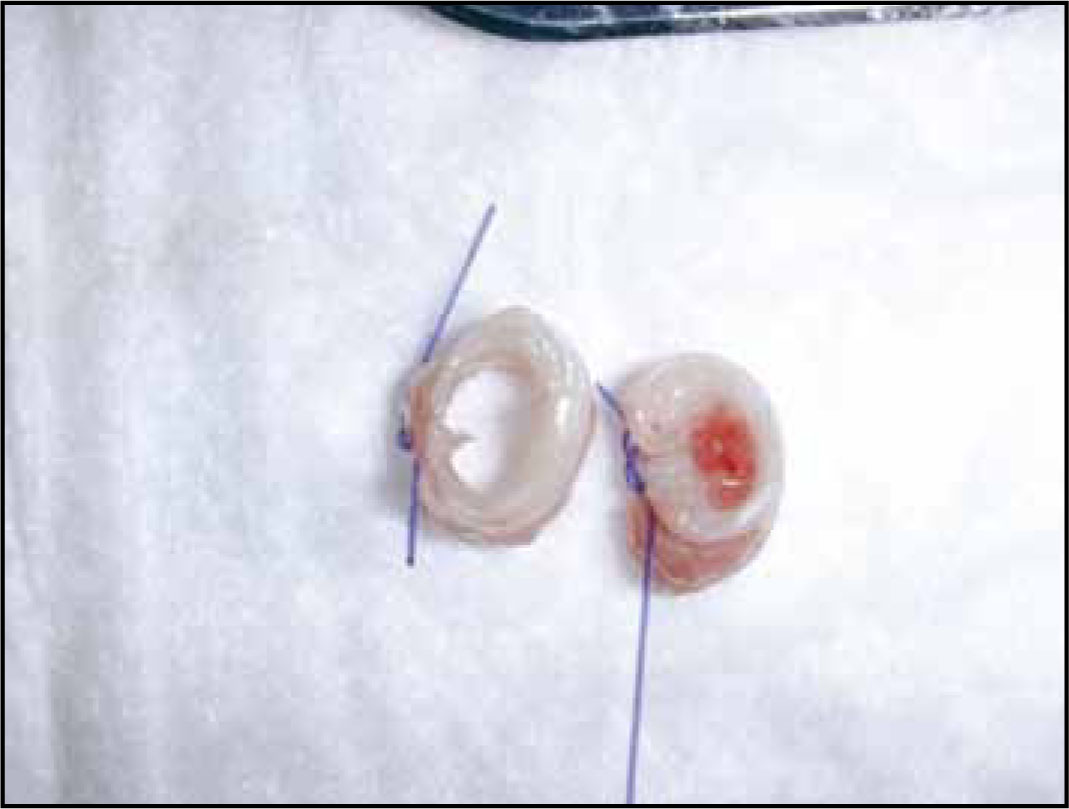
Due to its influence on capillary perfusion pressure, the systemic blood pressure of the patient should also be considered. Even with acceptable lateral wall pressure created by the ET tube cuff, signifcant reduction in mean arterial blood pressure (<50 mmHg) can result in a fall in tracheal blood flow (Inada et al, 1995). In the face of a high lateral wall pressure created by over inflation of the ET tube cuff, hypotensive periods can result in marked reduction in tracheal blood flow (Bunegin et al, 1993), placing the trachea at risk of ischaemic damage. The latter can lead to tracheal necrosis and possible tracheal stenosis (Figure 3). In light of such findings, it is essential that equal consideration is given to cuff inflation and blood pressure monitoring during anaesthesia. Ideally mean blood pressure should be maintained above 60 mmHg in the anaesthetized patient (Reuss-Lamky, 2010).
Current research investigating tracheal injury in cats
Hardie et al (1999) conducted three separate research studies to investigate tracheal injury related to cuffed ET tube intubation in the feline patient. Both retrospective clinical and experimental research approaches were utilized. The retrospective clinical study examined medical records of cats with a history of tracheal damage following an anaesthetic event wherein cuffed ET tubes had been utilized. Common characteristics between these cats were identified and categorized with the aim to highlight possible causes of tracheal injury. Sixteen cats were identified within this study. Their ages ranged from 2–16 years, suggesting that cuffed ET tubes can cause tracheal damage in cats of all ages. There was also no obvious breed or sex predisposition. The study did indicate that injury most commonly occurred following procedures where there was a risk of aspiration of fluid, such as dental or oral procedures (12/16 cats had undergone dental procedures, 3/16 bronchoalveolar lavage and the remaining 1/16 excision of a malignant histiocytoma). The postulated cause for the injury relating to dental procedures was over-zealous inflation of the ET tube cuff with the aim to avoid inhalation of dental fluid. This was suggested because during bronchoscopic examination a high number of cats (9/16), all of which had received dental treatment, had confirmed ruptures in a location consistent with cuff inflation. The remaining (7/16) cats received conservative management consisting of oxygen therapy and cage rest and bronchoscopic examination was not performed. The cause of injury in these cats was therefore not investigated. Cuff over inflation as the most likely cause of tracheal injury was later investigated within the experimental study discussed below.
The experimental study was conducted to examine potential mechanisms that could cause damage of the tracheal mucosa. This approach utilized two cat cadavers. Deliberate attempts were made to recreate tracheal injury that had been reported in the affected cats in the retrospective study noted above. Improper placement of an ET tube, use of an ET tube of improper size, over inflation of the ET tube cuff, improper use of an intubation stylet and failure to deflate the ET tube before repositioning or extubating the cadaver were all mechanisms examined. The results indicated that over inflation of the ET tube cuff was the only mechanism that could result in complete tracheal rupture, thus supporting the findings of the retrospective study noted above. The use of an intubation stylet did, if used inappropriately extending out past the lumen of the tube, penetrate the trachealis muscle and create a jagged hole, and repositioning the patient without disconnection from the anaesthetic circuit resulted in a 360° twist of the ET tube and subsequently the trachea. Both these mechanisms in the cat cadavers did not result in rupture of the tracheal mucosa. However, the effects of these mechanisms are still undesirable and should be avoided. Unfortunately the results for other mechanisms were not discussed.
Another study conducted by Hardie et al (1999) also investigated ET tube cuff design and the effect this had on tracheal injury. Ten cat cadavers were used and two cuff designs were examined, namely low pressure, high volume (LPHV) and HPLV. The reader is referred to the section Cuff design and effect on tracheal injury for further information on LPHV and HPLV cuff design. The results of this study indicate that there is no significant difference between the two cuff designs and both, if over inflated, did cause rupture of the tracheal mucosa. The only signifcant difference was that the LPHV cuffs if over infated did result in a longer tracheal tear (1.7–3.5 cm) compared with the HPLV cuff (1.5–1.6 cm), possibly because they have a larger cuff diameter (Hardie et al, 1999).
The studies conducted by Hardie et al (1999) are beneficial and provide some interesting insight demonstrating potential causes of tracheal injury in the feline patient. The only concern is that the use of cadavers does not allow a true representation of tracheal injury in live animals. Cat cadavers were obviously used for ethical reasons. However, living healthy animals are constantly moving and their tracheas are exposed to additional forces, for example coughing and sneezing. It is therefore fair to ask the question: could mechanisms of injury, for example the jagged hole created by the intubation stylet, later lead to a tracheal tear in a healthy moving animal? In addition, does an experimental study using only two cat cadavers give a true representation of the feline population? Thus suggesting that the mechanisms examined, i.e. multiple repositioning without cuff deflation, could potentially cause tracheal injury in patients with more delicate tracheas. The author's experience from clinical practice would support the view that multiple re-positioning during dental procedures coupled with an excessively inflated cuff could be a plausible explanation for tracheal injury.
The last point to consider is that unfortunately the design of the Hardie et al (1999) study does not investigate the cuff pressure exerted onto the tracheal mucosa. This study, therefore, does not take into account, when comparing the HPLV with the LPHV cuffs, the effect the cuff design has on perfusion pressure within the tracheal mucosa. LPHV cuffs have been specifically designed to reduce high focal points of pressure and instead spread the cuff pressure over a wider area of the trachea (Figure 4). The aim of the LPHV design is to prevent instances of high focal pressures that could lead to a reduction in capillary blood flow and subsequently damage to the trachea. Unfortunately however this is not investigated within the Hardie et al (1999) study.
Mitchell et al (2000) conducted a retrospective study to ascertain clinical features of cats with tracheal rupture associated with cuffed intubation. They identified 20 cats that had a history of tracheal trauma while receiving inhalation anaesthesia wherein a cuffed ET tube had been utilized. Their findings support those of Hardie et al as a high number (14/20) of the cats had undergone anaesthesia for dental treatment. The remaining (6/20) had undergone oral surgery for mass removal, neutering and radiographic procedures. One major difference is that this study did not investigate the possible mechanisms of injury, which remain unknown. Cuff over inflation was, however, deemed as the most likely cause of trachea rupture given the location of injury within the trachea. In addition, in 2/20 of the cases, cuff over-infation was admitted by the veterinary surgeon involved. In one case the vet admitted to inadvertently inflating the ET tube cuff for a second time after not realizing the cuff had been already inflated by another practitioner. This resulted in a popping sensation as the cuff ruptured through the patient's trachea. In another case a continual air leak around the ET tube cuff was reported even after adequate cuff inflation had been performed. Cuff inflation continued and the air leak was later found to be in another part of the ET tube. Two days later the cat underwent surgery to repair a tracheal tear.
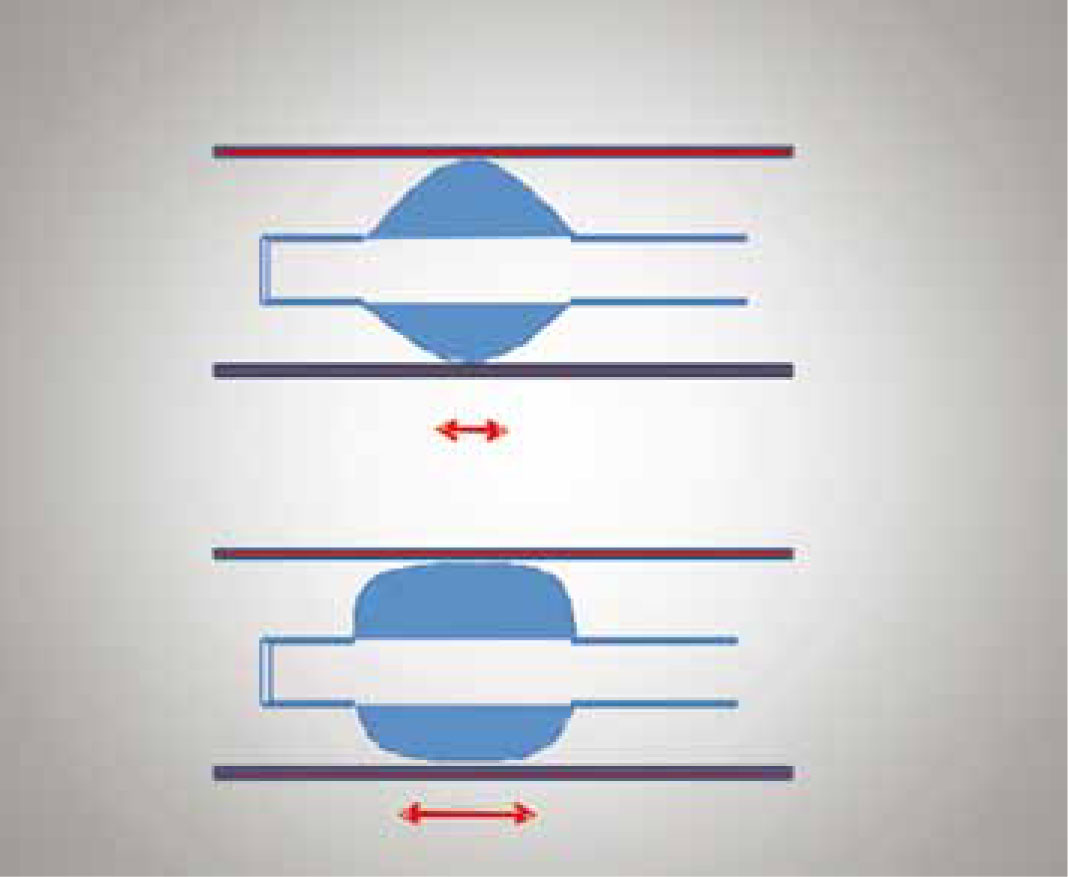
Mitchell et al (2000) also investigated the technique used by veterinarians when performing cuff inflation. This was achieved via a questionnaire that was sent to all the veterinary surgeons involved in the above study. 18/20 of the veterinarians replied and the results revealed that a high number (10/18) had inflated the ET tube cuff by feel only (until the operator experienced resistance to further inflation and the pilot balloon appeared inflated), 3/18 by the ‘minimal sound occlusion technique’ (until pressure on the reservoir bag did not result in an audible air leak escaping around the ET tube cuff) (Figure 5), and 5/18 by sound and feel combined. The high percentage of ruptured tracheas as a result of the ‘feel only’ technique demonstrates that use of this technique placed the patient at high risk of tracheal damage. The feel only technique in human practice has also been associated with high cuff inflation pressures (Janossy et al, 2010). This technique should therefore be avoided. Similarly, the minimal occlusion technique also resulted in tracheal rupture, suggesting that if used inappropriately, this too can have adverse sequalae that can result in rupture of the tracheal mucosa. Again, in human practice the minimal occlusion technique has been associated with excessive cuff inflation pressures (Kumar and Hirsch, 2011).
The results of Mitchell et al's study provide some valuable information highlighting causes of tracheal damage in feline patients. In addition, this study supports the findings of Hardie et al which indicated that tracheal damage occurs more often in patients undergoing dental procedures. One aspect of the Mitchell et al study that is especially beneficial is the investigation exploring current techniques of cuff inflation, which highlights that in veterinary practice an optimal technique for ensuring appropriate cuff inflation pressures in the feline trachea is lacking. In human practice, an alternative method of cuff inflation is to use a hand held pressure gauge (Figure 6) as a safer alternative to ensure an appropriate cuff inflation pressure (Ganner, 2001). The use of pressure gauges could be a potential benefit, allowing accurate cuff inflation pressures to be achieved in the feline patient. However, pressure gauges are expensive and are not available to every veterinary practitioner and even if gauges were available there appear to be limited data regarding safe cuff inflation pressures in the feline patient. Canine inflation pressures are however available. Instead, in veterinary medicine, the minimal occlusion technique appears to be the currently recommended cuff inflation technique (Hughes, 2007; Johnson, 2009). In clinical practice, the author of this article will continue to use the minimal occlusion technique until an optimal technique of cuff inflation has been determined. However, the minimal occlusion technique is not 100% safe and should therefore still be performed carefully. Box 1 contains some clinical recommendations for use of the minimal occlusion technique in practice.

Cuff design and effect on tracheal injury
Currently in veterinary medicine two types of ET tube cuff design are available: LPHV and HPLV (Figure 1). LPHV cuffed ET tubes are often clear and have graduated numbers along their length, while HPLV cuffed ET tubes are commonly opaque red rubber. In human medicine in the late 1960s the transition from HPLV to LPHV cuffs significantly reduced the incidence of tracheal complications (Hoffman et al, 2009). In veterinary medicine, both cuff designs continue to be used.
HPLV cuffs require small volumes of air to inflate the cuff but create great pressure over a small area of the tracheal mucosa. In effect, they close the dead space between the cuff and the trachea by exerting a lot of pressure (using a small volume of air) over a small area of the tracheal mucosa. Unfortunately this design may increase the risk of pressure necrosis and tracheal injury because the cuff pressure is not evenly distributed but instead is concentrated on an isolated area of the tracheal mucosa (Figure 4). This can then lead to tissue necrosis because the pressure exerted by the cuff impedes tracheal mucosal blood flow. Interestingly it is this cuff design that was used in all 11 cases of tracheal rupture reported to the VDS.
In contrast, LPHV cuffs require larger volumes of air to inflate the cuff and although this might seem counterintuitive, the larger volume of air and the design of the cuff allow for the pressure to be distributed more evenly over the tracheal mucosa (Figure 4). LPHV cuffs are preferable as the lower pressure caused by more even distribution of air is less likely to result in pressure necrosis and tracheal injury (Whitford and Johnsen, 2007). However, one problem associated with LPHV cuffs is that the cuff, even when adequately inflated, does not completely prevent fluid leakage into the lower airway (Asai and Shingu, 2001). The cuff diameter is greater than that of the trachea and even when inflated there are longitudinal folds that run down the side of the cuff, allowing some passage of fluid into the lower airway. In the author's experience this does not appear to be clinically relevant, since gross aspiration of fluid is prevented. When a procedure carries a high risk of fluid aspiration the use of throat packs or pharyngeal swabs is advisable to reduce aspiration. Some practitioners suggest coating the ET tube cuff with water-soluble gel (KY jelly) as an effective way to reduce leakage of fluid by plugging the longitudinal folds (Blunt et al, 2001). However, care should be taken, as ET tube obstruction has been reported (Badrekumer et al, 2001). Gel lubrication should therefore be used cautiously especially in patients with small tracheae, and if used the gel should not be allowed to enter the lumen of the tube.
As mentioned earlier, LPHV cuffs can result in a larger tracheal tear in comparison to the HPLV tube due to the larger cuff size (Hardie et al, 1999). This is another disadvantage of LPHV cuffs. However specifically designed paediatric ET tubes with a smaller cuff size and pilot balloon are now available (Figure 1). These tubes are ‘low contour’ tubes and exert low pressure on the tracheal mucosa.
Conclusion
In conclusion, tracheal trauma can occur as a direct result of cuffed ET intubation in the feline patient. The cause of tracheal injury is primarily iatrogenic and is a combined result of a delicate small trachea, over-zealous cuff inflation and poor ET tube management. It is therefore suggested that every practitioner undertaking cuffed ET intubation, especially in the feline patient, should make every effort to safeguard against tracheal injury. The aim of cuff inflation is to achieve a seal between the cuff and the trachea with a pressure great enough to prevent aspiration but not so high that tracheal blood flow is impeded.
There is limited research investigating causes of tracheal injury in the cat. Additional research in this area would be beneficial. However, the research that has been conducted demonstrates that tracheal injury most commonly occurs during dental procedures. Tracheal injury in such instances has been primarily linked to over inflation of the ET tube cuff. However, inappropriate use of an intubation stylet, twisting and repositioning the patient without careful management or deflation of the ET tube cuff should also be considered as a potential cause of injury. The author suggests a potential method of reducing tracheal injury during dental procedures is the use of throat packs and pharyngeal swabs to reduce aspiration of fluid and debris, and suction to actively remove dental fluid to avoid aspiration. Perhaps this will help avoid over-zealous cuff inflation with the aim to avoid aspiration of dental fluid.
It is also interesting that HPLV red rubber ET tubes had been used in all 11 cases of tracheal damage reported to the VDS. This tube design should therefore be used with caution. More research does need to be conducted within this area but, as indicated within human literature, after the introduction of LPHV cuffs the incidence of tracheal complications significantly reduced (Hoffman et al, 2009). Perhaps a safer alternative would therefore be to use the LPHV cuff design.
Hopefully, in the future of veterinary medicine, appropriate cuff inflation pressures will be investigated and an optimal technique of cuff inflation will be developed, thus making cuffed ET intubation safer in the feline patient.
