References
Managing nausea in the hospitalised patient
Abstract
Nausea is an unpleasant sensation of needing to vomit, and there are numerous negative consequences for the patient if not effectively managed. The clinical signs of nausea can vary widely from subtle to severe and, currently, there is no validated nausea scale for cats and dogs. The management of nausea often requires a multimodal approach, including management of the underlying disease process and pharmacological approaches to minimise the unpleasant sensation. Registered veterinary nurses should have an understanding of the mechanisms of nausea, as this guides drug selection, and also of the pharmacology of each of the anti-nausea medications.
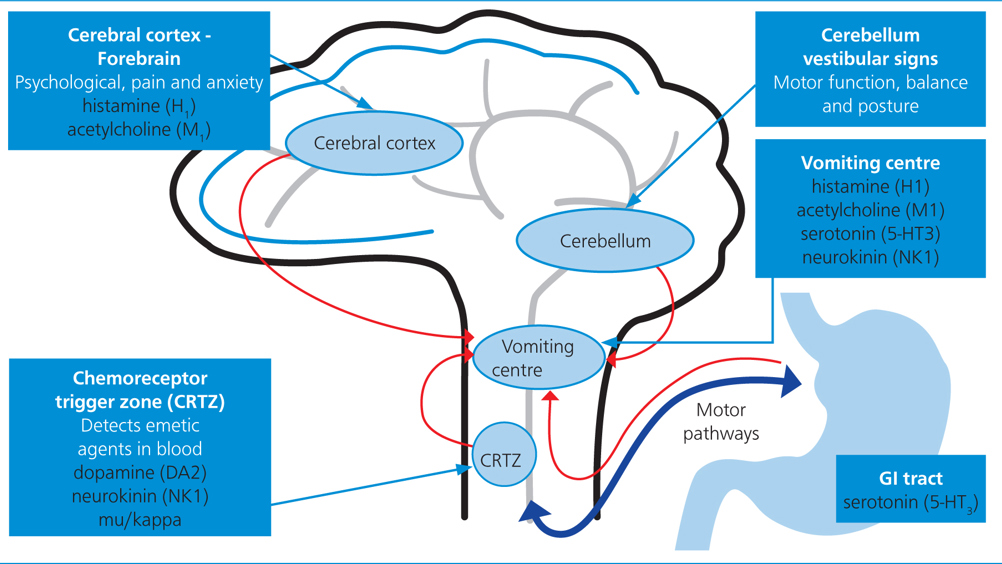
Nausea is described as the unpleasant sensation of needing to vomit, while vomiting or emesis is the forceful oral expulsion of gastrointestinal (GI) contents. While vomiting may have a protective mechanism by removing potential harmful substances, nausea can result in significant debilitation and impact patient welfare. The possibility of nausea in an owner's cat or dog can result in owner distress and most owners are willing to pay for additional treatment for the clinical signs of nausea (Hay Kraus and Cazlan, 2019).
Nausea does not always result in vomiting, and is believed to be more challenging to manage than the act of vomiting (Morrow et al, 2002; Topal et al, 2005). There are numerous causes of nausea such as postoperative and anaesthesia-associated drugs including opioids and chemotherapeutics, and conditions affecting the brain, vestibular system and abdominal viscera. However, nausea is a subjective individual event and, while it is associated with numerous clinical signs, it may be difficult to truly interpret its full impact on a particular patient. Vomiting should not be misinterpreted as regurgitation, and therefore regurgitation will also be discussed. Registered veterinary nurses play a key role in identifying nauseous patients, administering antiemetics and providing additional supportive measures to decrease nausea in hospitalised patients.
Register now to continue reading
Thank you for visiting The Veterinary Nurse and reading some of our peer-reviewed content for veterinary professionals. To continue reading this article, please register today.

