Uroabdomen refers to the presence of urine in the abdomen, which occurs secondary to a rupture in the urinary tract. Urine leakage accumulates in the peritoneal or retroperitoneal cavity (or both), resulting in an effusion (Valtolina, 2018). Uroperitoneal effusion is most commonly seen in dogs and cats, with multiple causes including trauma, following obstruction, iatrogenic damage from urinary catheter placement or the presence of neoplasia (Stilwell, 2017; Press and Balakrishnan, 2018). Uroperitoneal effusions occur secondary to a rupture in the lower urinary tract, i.e. the urethra, urinary bladder or distal ureter, whereas a uroreteroperitoneal effusion occurs with injury to the kidney or proximal ureter (Stilwell, 2017; Foster and Humm, 2018).
Hypovolaemia and electrolyte and metabolic disturbances occur secondary to a uroabdomen, with derangements including hyperkalaemia, azotaemia and metabolic acidosis. Prompt stabilisation is required to prevent multisystemic effects becoming life threatening (Stafford and Bartges, 2013; Stilwell, 2017).
This article will explore the nursing of the uroabdomen patient, from initial presentation and stabilisation through to diagnosis and surgical intervention.
Aetiology
A uroabdomen may occur following blunt trauma to the abdomen or pelvis, such as a road traffic accident (RTA). Trauma to any part of the urinary system can result in a rupture, with damage to the urinary bladder and urethra often seen in small animal practice (Foster and Humm, 2018). Trauma is less likely to cause a ureteral rupture because of the relatively small size and mobility of the ureter in dogs and cats (Stafford and Bartges, 2013). It is reported that bladder rupture incurred by trauma is the most common cause of a uroabdomen in young male dogs, possibly because of their roaming behaviour resulting in a higher incidence of RTAs (Mayhew and Holt, 2004; Grimes et al, 2018).
Iatrogenic causes of a uroabdomen often involve trauma to the bladder or urethra. Urethral tearing commonly occurs secondary to urinary catheter placement. A uroabdomen is evident if the bladder is no longer palpable or fails to distend when filled — either a bladder rupture or urethral tear has taken place (Thomovsky and Plunkett, 2013; Foster and Humm, 2018). Manual expression of the distended bladder may result in a uroabdomen through bladder rupture, particularly in the urethral obstruction patient (Foster and Humm, 2018). This is commonly seen in male cats that are predisposed to obstruction because of their long narrow urethra and stress-related urinary behaviour (Colopy and Bjorling, 2016; Rademacher, 2019). Uroliths can also be found in the kidney and ureter (Stilwell, 2017).
Other iatrogenic causes of a uroabdomen include bladder injury from cystocentesis or accidental lacerations during surgery (Stafford and Bartges, 2013; Stilwell, 2017).
Initial assessment and diagnosis
On arrival to the practice a capsule history should be taken from the owner and consent for treatment gained. The patient's resuscitation code should be discussed, as the uroabdomen patient can be critical on presentation. An initial triage assessment should include evaluation of the major body systems, followed by a full physical examination. Assessment of pain should take place at this time so that appropriate analgesia may be administered (Thomas and Lerche, 2017). Urine is a chemical irritant, making a uroabdomen very painful (Stilwell, 2017).
Clinical parameters should include assessment of mentation, respiratory rate and effort, heart rate, pulse quality, mucous membrane colour and moistness, capillary refill time and temperature, with close attention paid to perfusion parameters (Stilwell, 2017). Other patient monitoring will ideally include a non-invasive blood pressure, electrocardiogram (ECG) and pulse oximetry, to assess perfusion, heart rhythm and ventilation (Colopy and Bjorling, 2016). The patient may be hypotensive if hypovolaemic or hypertensive with an acute kidney injury (AKI) (Foster and Humm, 2018).
The uroabdomen patient may present to the veterinary practice in a critical condition, so diagnostic workup should take place alongside stabilisation. In the author's practice a blood sample is taken directly from a newly placed intravenous catheter to maximise efficiency and minimise venepuncture to the patient. This also allows blood results to be analysed before the implementation of intravenous fluid therapy (IVFT), which will alter results by improving hydration and perfusion (Donohoe, 2016). Initial blood tests should include a minimum database of electrolytes, packed cell volume (PCV), total solids (TS) and a blood gas analysis if available. Haematology and biochemistry panels may also be performed (Sabino et al, 2016; Sabino, 2017). The severity of the patient's condition and the severity of bloodwork derangements are dependent on how long the uroabdomen has been present (Stafford and Bartges, 2013; Colopy and Bjorling, 2016).
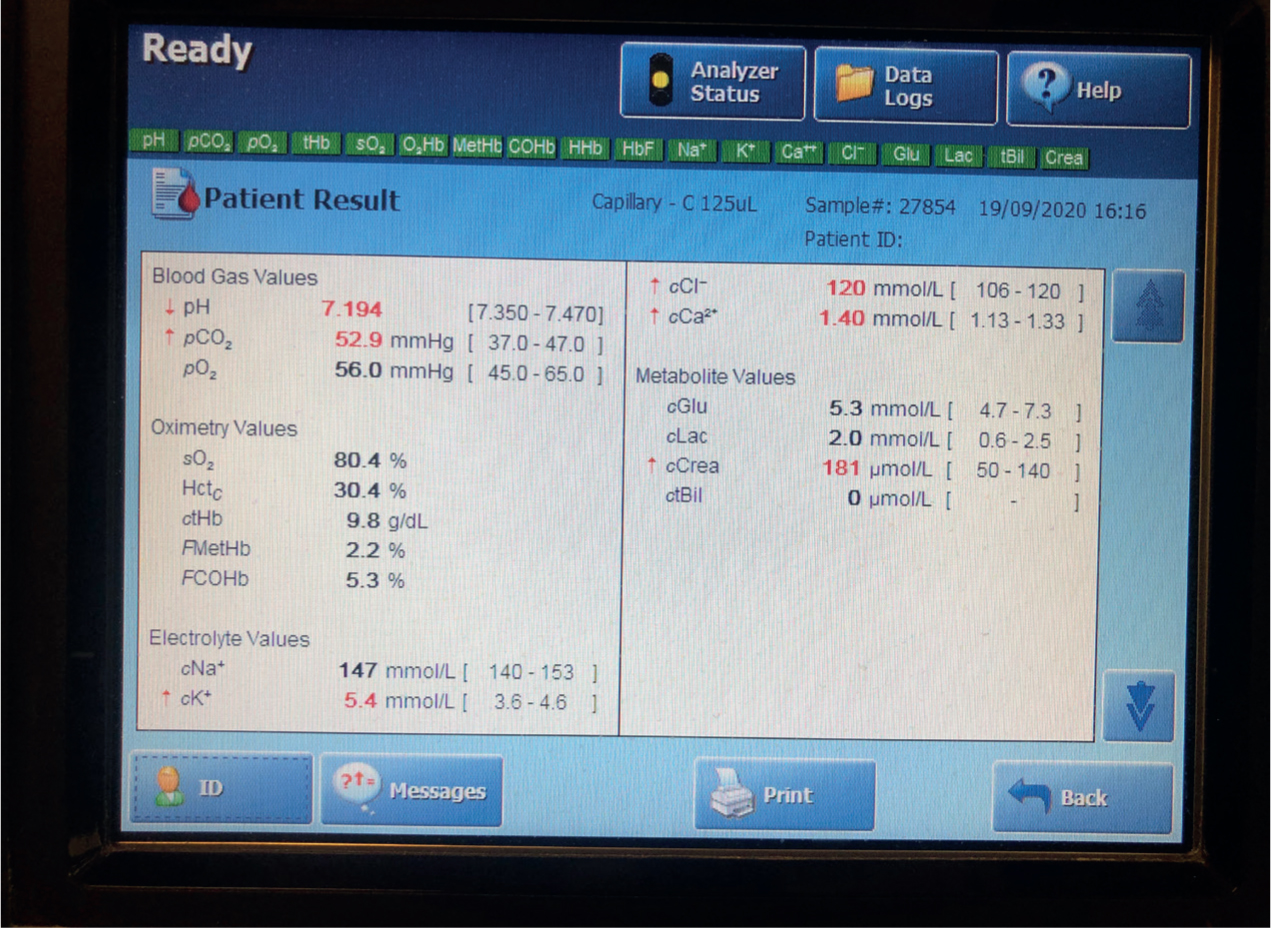
Hyperkalaemia and azotaemia are indicative of a uroabdomen, particularly in the trauma patient. Both abnormalities occur as the kidneys are unable to excrete potassium, urea and creatinine via urination (Stilwell, 2017; Foster and Humm, 2018). As urine accumulates in the peritoneal space, potassium, urea and creatinine are absorbed from the urine back into systemic circulation, causing serum levels to be elevated. The elevated urea and creatinine are referred to as post-renal azotaemia and can be life threatening if severe (Shumacher, 2016, Press and Balakrishnan, 2018).
Hyperkalaemia is a critical electrolyte imbalance with deleterious effects on ventilation, cardiac contractility, blood pressure and neuromuscular function (Orme, 2015; Colopy and Bjorling, 2016; Barton and Kirby, 2017). When interpreting the ECG trace, bradycardia, tall T-waves and wide QRS complexes are seen with mild to moderate hyperkalaemia (Figure 1). As hyperkalaemia becomes severe, P waves are progressively flattened and atrial standstill and ventricular fibrillation leading to asystole may occur (Stilwell, 2017; Valtolina, 2018).
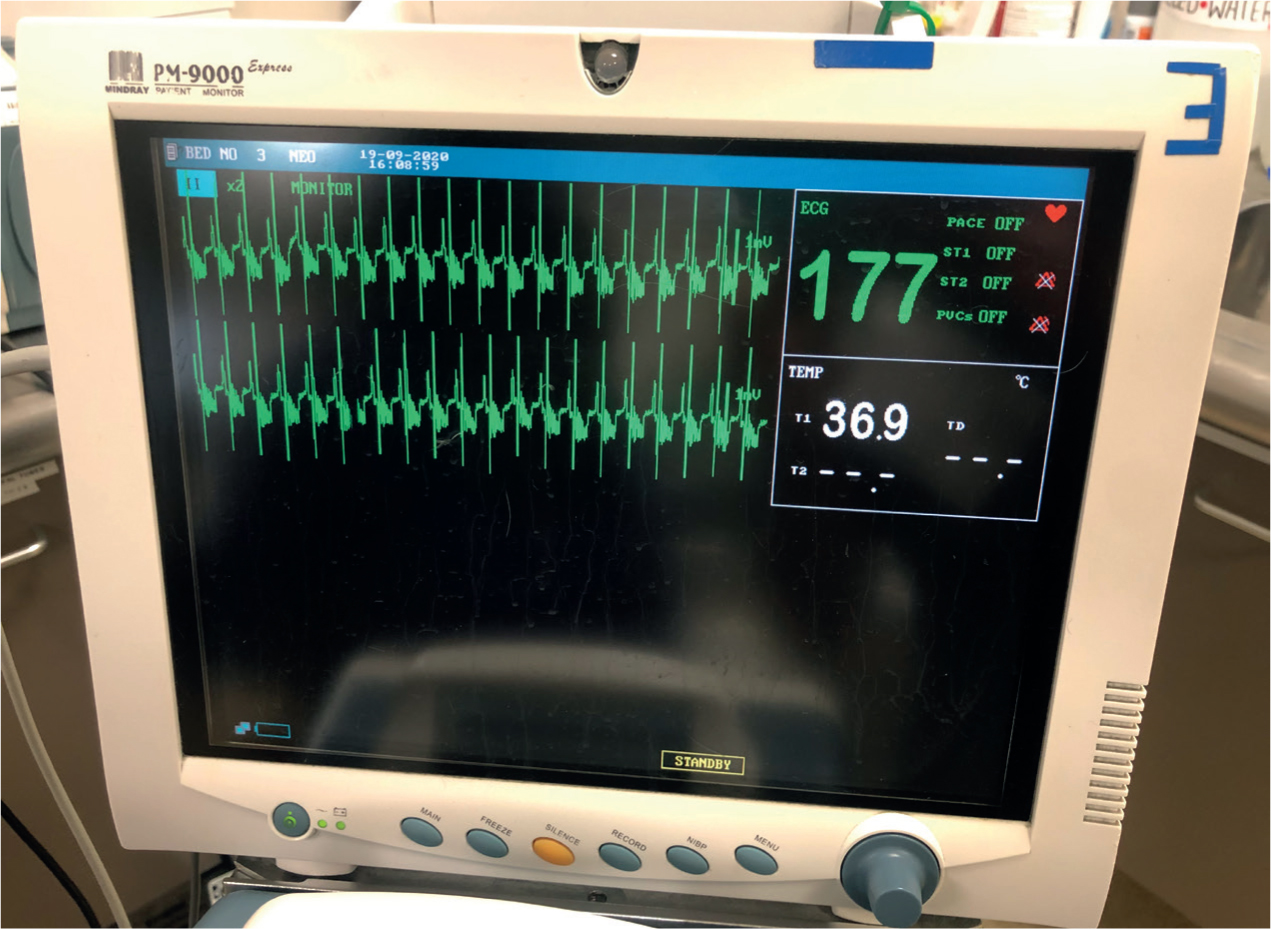
Hypovolaemia is seen with a uroabdomen as large particles in the urine cause water to be drawn out of the intracellular and intravascular spaces (Stilwell, 2017). The patient is in hypovolaemic shock when this reduction in circulating blood volume causes hypoperfusion (O'Dwyer and Girling, 2020). Blood gas analysis allows for interpretation of blood gases and pH (Figure 2). Metabolic acidosis is the major metabolic derangement seen in the uroabdomen patient, as the kidneys are unable to excrete hydrogen ions. The resulting renal hypoperfusion causes hyperlactataemia which worsens acidosis. Metabolic acidosis also contributes to hyperkalaemia (Orme, 2015; Sabino, 2017). Dehydration may be present if vomiting has occurred (Valtolina 2018).
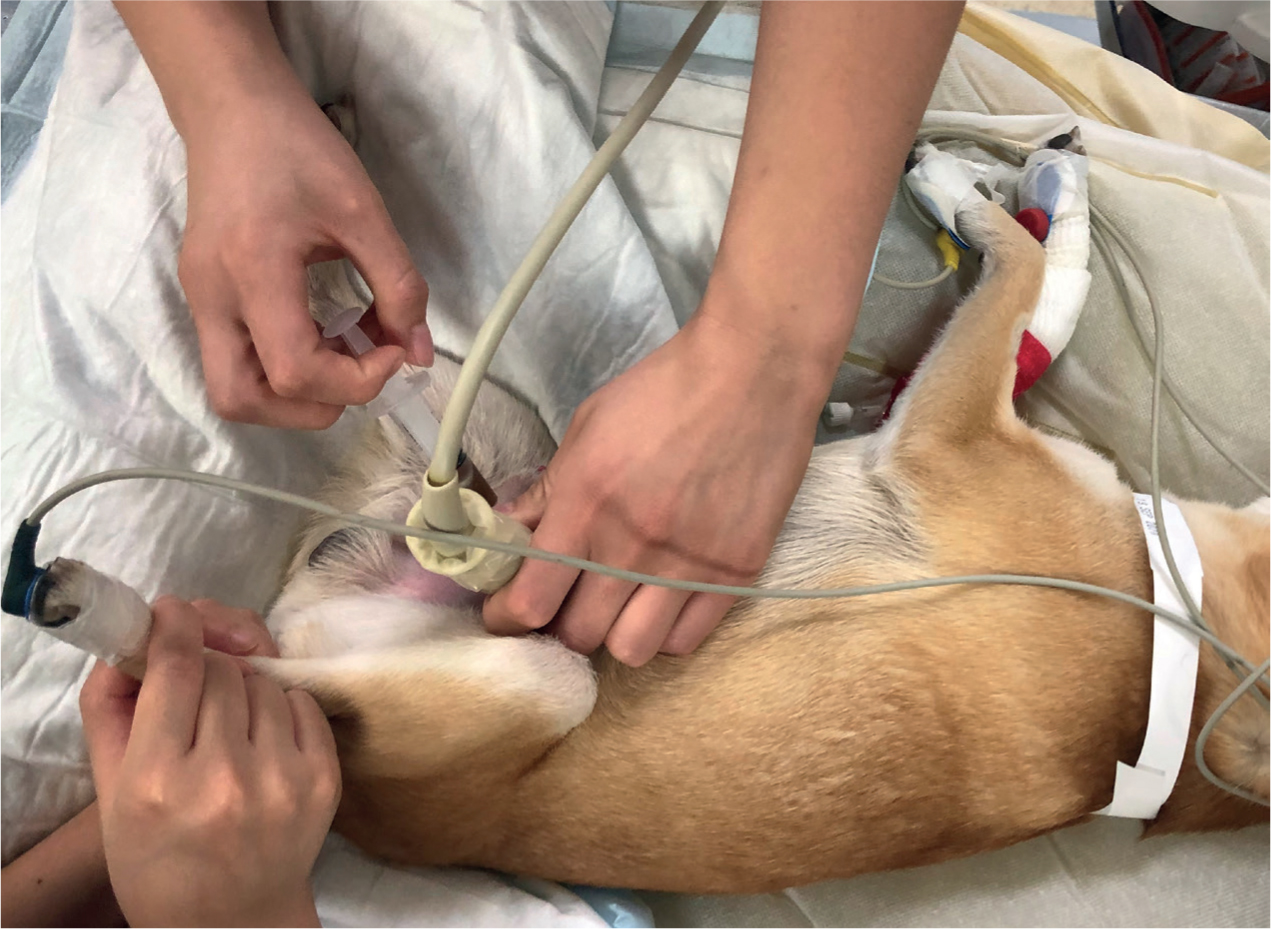
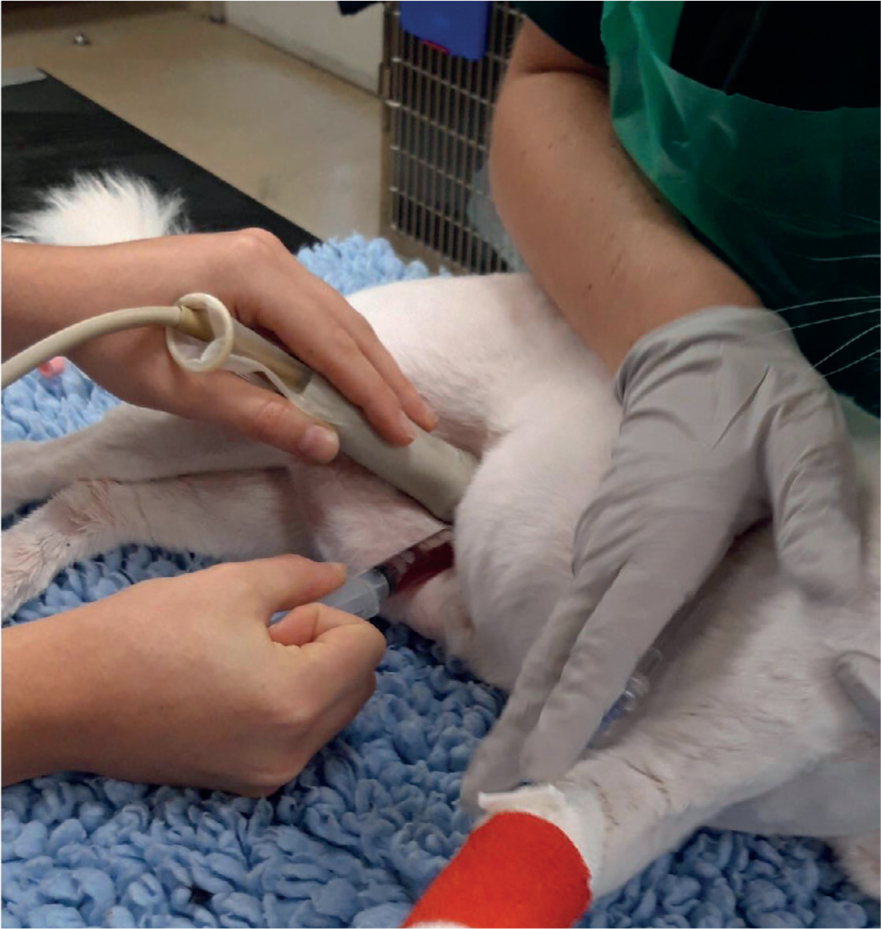
Peritoneal effusion can be identified with point of care ultrasound (POCUS), which can be performed during the physical examination. This is referred to as an AFAST (abdominal focused assessment sonography for trauma) if trauma-related (Valtolina, 2018). To definitively diagnose a uroabdomen the effusion must be identified as urine, so a sample needs to be taken. Abdominocentesis should be performed, which can be done with or without ultrasound guidance (Foster and Humm, 2018). This is a minimally invasive procedure, which can be performed conscious, with the patient restrained in left lateral recumbency to prevent splenic damage (Jandrey, 2014; Colopy and Bjorling, 2016) (Figures 3 and 4).
The fluid should be analysed, and the concentration of potassium and creatinine compared with serum potassium and creatinine levels. Though urea is also present in peritoneal fluid, it is an unreliable marker for diagnosis as the small particles can easily pass across the peritoneum (Stafford and Bartges, 2013; Colopy and Bjorling, 2016). A higher concentration of potassium and creatinine in peritoneal fluid than in serum is suggestive of a uroabdomen, with an increase in the ratio increasing the likelihood (Stafford and Bartges, 2013; Foster and Humm, 2018). In recent literature the concentration of potassium required for a diagnosis ranges from 1.4:1 to 1.9:1 (Herold, 2017; Klaus, 2017; Stilwell, 2017). Over twice the concentration of peritoneal fluid to serum creatinine is required for confirmation of a uroabdomen (Jandrey, 2014; Colopy and Bjorling, 2016). See Figure 5 for comparison of peritoneal fluid and serum concentrations.
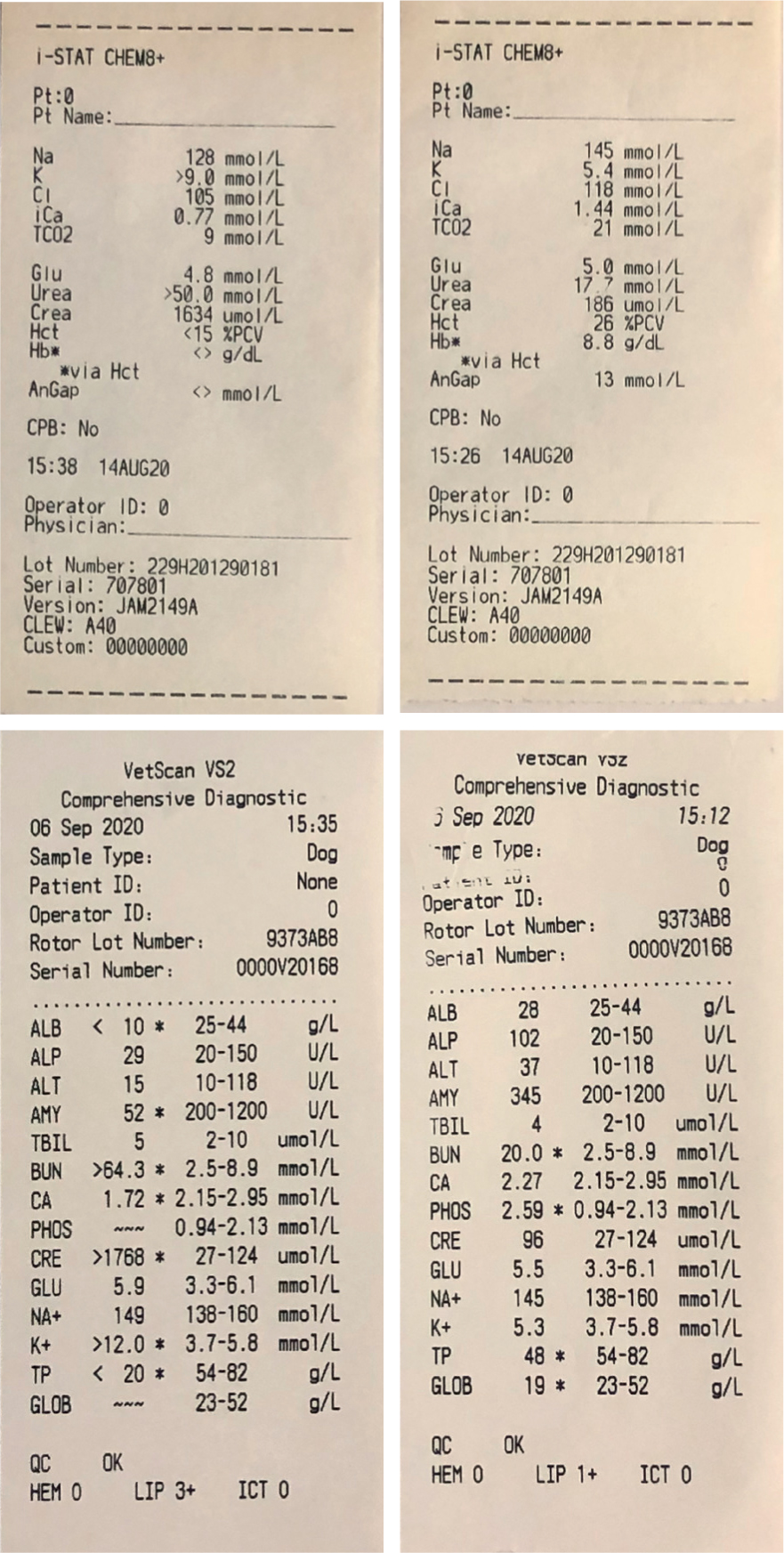
In addition to potassium and creatinine testing, peritoneal fluid should be cytologically analysed; septic peritonitis can occur secondary to a urinary tract infection prior to the uroabdomen. If septic, then fluid samples should be sent for culture and sensitivity testing to ensure appropriate antimicrobial selection (Stafford and Bartges, 2013; Colopy and Bjorling, 2016; Stilwell, 2017).
Clinical history and presentation
On presentation to the veterinary practice, diagnosis of a uroabdomen may not occur immediately, particularly in the trauma patient with concurrent injuries (Valtolina, 2018). Presence of a uroabdomen may be suspected based on the clinical presentation and patient history, but is not confirmed until laboratory evaluation of peritoneal effusion confirms the effusion contains urine (Colopy and Bjorling, 2016; Stilwell, 2017). If trauma such as a RTA has occurred, then a uroabdomen should be suspected, particularly with abdominal or hind limb injury such as rib or pelvic fractures, or if there is the presence of haematuria (Colopy and Bjorling, 2016; Stilwell, 2017; Foster and Humm, 2018). Although anuria or haematuria may be present, some patients will be able to urinate normally despite damage to the urinary tract (Foster and Humm, 2018; Boag and Marshall, 2020).
On presentation, physical examination may include a distended or painful abdomen, with a depressed mentation, bradycardia or tachycardia with or without arrythmias. Clinical history may include non-specific signs such as anorexia, vomiting and weakness. Such clinical signs occur secondary to hypovolaemia, hyperkalaemia and azotaemia (Stafford and Bartges, 2013; Stilwell, 2017; Boag and Marshall, 2020). Trauma patients may still have a palpable bladder despite a bladder rupture, so the ability to palpate the bladder does not mean it is intact (Valtolina, 2018).
Stabilisation
Analgesia
Provision of analgesia should occur during stabilisation as prescribed by the veterinary surgeon, to reduce pain and stress, which can contribute to patient deterioration (Sabino et al, 2016; Sabino, 2017). Analgesia may be administered intramuscularly before continuing with other stabilisation procedures to ensure patient comfort and good patient welfare. If required, supplementation with oxygen therapy should also be made a priority at this time. Placement of a peripheral intravenous catheter should be performed when appropriate to facilitate IVFT, administration of emergency drugs if required and further doses of analgesia (Thomovsky and Plunkett, 2013; Cooper and Roasa, 2017).
Administration of μ-agonist opioids, such as methadone or fentanyl, are recommended for their potent analgesic properties (Cooper and Roasa, 2017; Thomas and Lerche, 2017; Valtolina 2018). Other benefits include the ability to be reversed if necessary, such as if the patient arrests (Stafford and Bartges, 2013). If these are unavailable then buprenorphine may be considered, but as a partial μ-agonist opioid it is less effective for the severe pain encountered with a uroabdomen (Stilwell, 2017; Thomas and Lerche, 2017).
Electrolyte and metabolic derangements
Hyperkalaemia is the most life-threatening electrolyte disturbance encountered by the uroabdomen patient and should be treated as a matter of urgency. Treatment options vary depending on the severity of hyperkalaemia; a mildly elevated potassium can be normalised with IVFT alone (DiBartola and Westropp, 2020). Other benefits of IVFT include improvement of azotaemia, which must be treated to prevent an acute kidney injury (AKI) through severe renal hypoperfusion (Foster and Humm, 2018). Administration of fluids improves renal perfusion and glomerular filtration rate (GFR), resulting in increased excretion of potassium, urea and creatinine (Stafford and Bartges, 2013; Stilwell, 2017).
Treatment of severe hyperkalaemia aims to minimise the cardiotoxic effects seen with a potassium level over 7.5 mmol/litre. Administration of calcium gluconate protects the myocardium; insulin and dextrose therapy may then be administered to stimulate uptake of potassium into cells. Following this redistribution, potassium may then be eliminated with IVFT (Stilwell, 2017; O'Dwyer and Girling, 2020). Glucose supplemented fluids may be required to counteract the longstanding effects of insulin (Valtolina, 2018).
Hypovolaemia should also be treated with IVFT, ideally an isotonic crystalloid to replace perfusion deficits. Fluids may be administered as boluses, as directed by a veterinary surgeon, with perfusion parameters closely monitored between boluses (Foster and Humm, 2018; O'Dwyer and Girling, 2020). If in hypovolaemic shock, oxygen therapy may also be indicated (Stafford and Bartges, 2013).
Compound sodium lactate is an isotonic crystalloid fluid which is effective at treating hyperkalaemia despite containing a small amount of potassium (Colopy and Bjorling, 2016; Valtolina, 2018). Compound sodium lactate also contains lactate, which acts as an alkalising agent and corrects metabolic acidosis (Stilwell, 2017; O'Dwyer and Girling, 2020). If acidosis is severe, or IVFT is ineffective, then alkalising therapy with sodium bicarbonate may be required (DiBartola and Westropp, 2020; Colopy and Bjorling, 2016).
Urinary diversion
Urine must be drained from the abdomen to treat the underlying cause of metabolic and electrolyte derangements; emergency stabilisation is futile if the effusion continues to accumulate and worsen hyperkalaemia and azotaemia. This can be a temporary measure performed in the conscious patient, alongside IVFT to aid stabilisation before a general anaesthetic (GA) and diagnostic imaging are considered (Colopy and Bjorling, 2016; Foster and Humm, 2018).
Options for urinary diversion include placement of a urinary catheter. This is effective if a bladder rupture is present as it decompresses the bladder and prevents ongoing leakage (Stafford and Barges, 2013; Foster and Humm, 2018). In the case of a simple urethral tear, the urinary catheter can bypass the tear and enable normal urinary flow (Valtolina, 2018).
The obstructed patient will require emergency deobstruction, but GA is high risk because of hypovolaemia and/or dehydration and the cardiotoxic effects of hyperkalaemia (Aldridge and O'Dwyer, 2013; Sabino et al, 2016). Therapeutic cystocentesis may be considered but comes with risk of bladder rupture as pressure inside the bladder is elevated.
If the patient has more complex urethral damage, a urethral blockage, or damage further up the urinary tract at the ureters or kidneys, then placement of a urinary catheter may not be effective. Surgical placement of a cystotomy tube may be advised, which requires a GA and carries risk of further complications, discussed below (Stafford and Bartges, 2013; Stilwell, 2017).
Drainage directly from the peritoneal space can be performed in the conscious patient by abdominocentesis. A peritoneal catheter is not always required but should be considered if urine leakage continues. Placement can be performed conscious with local anaesthetic, or surgically if it needs to be in situ for several days (Stafford and Bartges, 2013; Foster and Humm, 2018). If stabilisation is ineffective then peritoneal dialysis may be required. This is currently provided in a small number of referral hospitals (Colopy and Bjorling, 2016; Stilwell, 2017).
Stabilisation of the patient to correct electrolyte abnormalities, azotaemia and perfusion deficits is essential for haemodynamic stability before a GA. Diagnostic imaging and any surgical repair needed should not take place until this has been performed (Colopy and Bjorling, 2016; Stilwell, 2017).
Diagnostic imaging
Following diagnosis of a uroabdomen and patient stabilisation, diagnostic imaging is necessary to confirm the location of the urinary tract rupture and enable surgical repair (Colopy and Bjorling, 2016). Several methods of imaging are available, with the selection made often dependent on availability and cost (Stilwell, 2017).
Ultrasonography
Most practices are equipped with an ultrasound machine. Kidney structure may be visualised during a full abdominal ultrasound and fluid may be seen leaking from the urinary tract, however this is dependent on user ability and experience. Ultrasound imaging is useful as described above for POCUS and initial identification of free fluid, but is rarely sufficient for localisation of the rupture (Stafford and Bartges, 2013; Herold, 2017).
Contrast radiography
Radiography is another readily available mode of imaging, however because of limited detail between types of soft tissue, survey radiographs are not diagnostic, and can only confirm the presence of a peritoneal effusion (Holloway, 2015; Valtolina, 2018). Contrast media can be used to increase the contrast between the urinary tract and surrounding tissues to highlight them for radiographs. When a tear is present this is demonstrated by leakage of contrast media into the peritoneal space (Colopy and Bjorling, 2016; Herold, 2017). Ideally, the patient would be fasted and have had an enema to prevent interference from digested food, however this is not possible with the emergency presentation (Rademacher, 2019).
Cystography is a fast and easy to perform imaging technique used to evaluate the integrity of the urinary bladder (Rademacher, 2019). A positive contrast cystogram uses a water-soluble iodine-based contrast media to coat the bladder (Holloway, 2015; Colopy and Bjorling, 2016). This is administered via a sterilely placed urinary catheter after the bladder has been emptied of urine. Abdominal radiographs are taken, and any contrast media leakages are identified. If a urethral tear is suspected, then a urethrogram should be performed. This is a similar process to the cystogram, except the urinary catheter is placed in the distal urethra and only the urethra is filled with contrast media (Stafford and Bartges, 2013; Rademacher, 2019). When performed together, the procedure is referred to as a urethrocystogram, and is often the imaging technique of choice as most urinary tract tears occur in the bladder and urethra (Stilwell, 2017; Foster and Humm, 2018).
Intravenous urography
Intravenous urography is a method of imaging used to assess the entirety of the urinary tract system, including the structure of the kidneys and ureters. This is preferred if the effusion is in the retroperitoneal space, or if other contrast studies have not revealed ruptures in the urethra or bladder (Rademacher, 2018; Valtolina, 2018). A water-soluble, iodine-based contrast media is administered via a peripheral intravenous catheter, then multiple radiographs are taken as it is excreted by glomerular filtration by the kidneys (Holloway, 2015; Rademacher 2019).
Use of intravenous contrast media comes with risks, as it is nephrotoxic and can cause contrast-induced renal failure (Holloway, 2015; Foster and Humm, 2018). The patient must have been stabilised, as pre-existing renal disease, azotaemia and dehydration exacerbate these side effects. The patient should be monitored closely as bradycardia and hypotension may also occur. Other less severe side effects include irritation, so the intravenous catheter should have been successfully placed on the first attempt (Holloway, 2015).
Surgery
If the location of the urinary tract rupture has not been found with diagnostic imaging, then an exploratory laparotomy may be performed to inspect for any leakage (Colopy and Bjorling, 2016). Medical management alone may be sufficient in cases when the rupture does not require surgical repair; simple urethral tears can be easily managed by placement of an indwelling urinary catheter left in situ for 1 to 3 weeks while the tear heals (Stafford and Bartges, 2013; Foster and Humm, 2018; Hornsey et al, 2020). More complicated urethral tears require a surgical cystotomy, where a catheter is placed directly into the urinary bladder, bypassing the urethra. This can be a temporary or permanent measure depending on the extent of damage (Stafford and Bartges, 2013; Foster and Humm, 2018).
Presence of a bladder rupture indicates that surgical repair is required. During surgery ruptures and lacerations are repaired and necrotic tissue is debrided. The abdomen is then drained and thoroughly lavaged to remove traces of urine (Stafford and Bartges, 2013; Foster and Humm, 2018). A cystotomy may be performed if the bladder rupture is severe.
Damage to the ureters requires surgical intervention as a matter of urgency; the small ureters in dogs and cats makes surgery difficult. The ureters can be reimplanted to the bladder, enabling urine flow (Stafford and Bartges, 2013; Foster and Humm, 2018). Major trauma to the ureter and kidneys may require surgical removal, i.e. a ureteronephrectomy (Foster and Humm, 2018).
Any surgical intervention comes with risk of complications, with the most common being wound breakdown. When repairing the urinary tract, this comes with the added risk of reaccumulation of a uroabdomen, or septic peritonitis if bacteria is present. Other risks include stricture of the urethra or ureter and urinary incontinence (Stafford and Bartges, 2013). Close monitoring in the postoperative period is essential for the identification of any surgical complications.
Nursing care
Nursing the uroabdomen patient utilises a multitude of the skills that registered veterinary nurses (RVNs) possess. During initial triage, nursing includes client communication when taking a capsule history and examining the patient to identify any urgent concerns. Practical skills include placement of an intravenous catheter, venepuncture and performing laboratory tests under the instruction of a veterinary surgeon during stabilisation. Patient restraint is necessary during procedures such as abdominocentesis and urinary catheterisation. The RVN may also perform more advanced practical skills such as urinary catheter placement.
The patient may be hospitalised for a considerable amount of time, so a quiet and calm environment and suitable bedding should be provided to ensure patient comfort. Making time to get to know the patient, regular walks if appropriate and giving tender, loving care (TLC) when treatments are not being performed can minimise stress and improve patient welfare (Darbo and Page, 2017).
Excellent nursing care is vital for patient recovery; the patient should be treated holistically, with a focus on overall wellbeing in addition to the concerns encountered by the uroabdomen patient. Use of a nursing care plan such as the Orpet and Jeffery Ability Model (2011) ensures that all aspects of nursing are addressed and can serve as a useful educational tool (Orpet and Welsh, 2011).
Anaesthesia and analgesia
Anaesthetic monitoring is required for diagnostic imaging and during any surgical procedures. Despite efforts to stabilise the patient, any sedation or GA carries risks and close monitoring is crucial. Assessment of pain is an important role of the RVN and should include routine use of a pain scoring system. This is vital on arrival to the practice and following any surgery when the patient might be painful, but should also be performed regularly as part of patient monitoring with use of a validated pain scoring assessment system (Thomas and Lerche, 2017).
Monitoring
The patient may be hospitalised in practice or sent to an out of hours provider for post-GA and post-surgical monitoring. Monitoring of clinical parameters should be performed regularly and recorded on kennel sheets, to include assessment of mentation, perfusion, cardiovascular stability, pain and signs of infection; temperature should be taken at least twice daily for detection of pyrexia (Shumacher, 2016; Stilwell, 2017; Ford-Fennah et al, 2020). Daily unbandaging and examination of the intravenous catheter is necessary to maintain patency and monitor for catheter-associated infection (Battaglia and Hirsh-Fitzpatrick, 2016). If surgery was performed, then the surgical site should be assessed and wound dressings changed at least daily (Anderson and Smith, 2020).
Regular blood tests should be performed to monitor previously discussed electrolyte and metabolic derangements and prevent patient deterioration. Blood glucose should also be monitored, particularly if insulin and dextrose therapy was administered or septic peritonitis occurred. A central venous catheter may be placed during surgery to allow fluid and drug administration and minimally invasive blood sampling. This improves patient welfare by reducing stress from venepuncture and handling.
Urinary catheter care and fluid balance
Indwelling urinary catheters must be managed appropriately to maintain cleanliness and reduce the risk of infection. A sign indicating the patient has a urinary catheter may be attached to the kennel door to ensure vigilance when handling the closed collection system, which should avoid contact with the floor (Figure 6) and be handled with gloves (Darbo and Page, 2017). Catheter care should include cleaning external lines with chlorhexidine solution to prevent ascending urinary tract infections (Battaglia and Steele, 2016). Care must be taken when moving the patient to minimise tension on the catheter and prevent disconnection or discomfort. Patient interference is common, so a buster collar should be worn (Darbo and Page, 2017; Ford-Fennah et al, 2020).
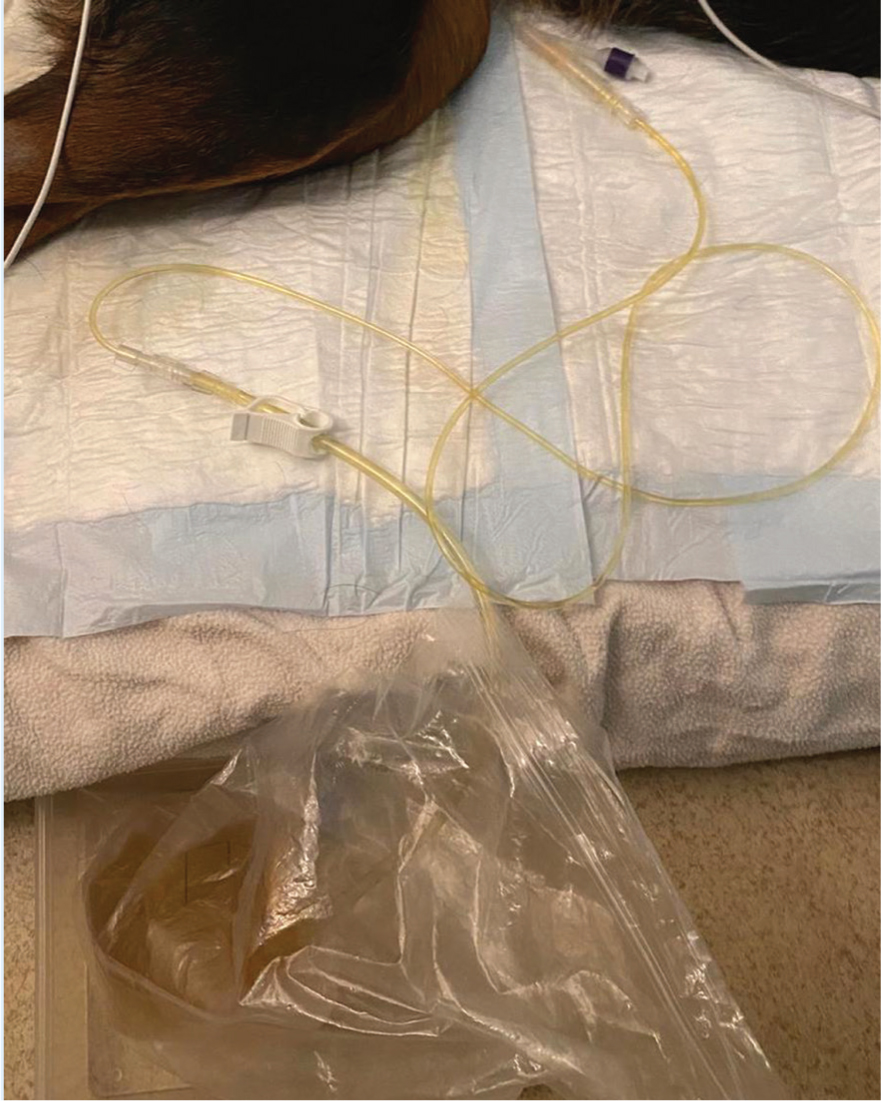
In addition to being necessary for urinary diversion, advantages of a urinary catheter include prevention of scalding from urinary incontinence and visual assessment of urine colour and turbidity (Foster and Humm, 2018; Mullineaux, 2020). The collection system should be emptied every 4 hours so that urine output may be calculated. If a urinary catheter is not in situ then incontinence sheets, bedding and litter should be weighed and used to estimate the volume of urine passed (Donohoe, 2016; Mullineaux, 2020). POCUS may be utilised to measure bladder size if there are concerns about urinary output. This is preferable to bladder palpation and may be performed by the RVN.
Urinary output is interpreted alongside urine specific gravity to assess renal perfusion and hydration status (Shumacher, 2016). Concerns should be reported to the veterinary surgeon; oliguria and an elevated urine specific gravity may indicate a recurring uroabdomen, impaired renal perfusion or dehydration (Colopy and Bjorling, 2016; Mullineaux, 2020). Other methods of assessing hydration status include weighing the patient at least twice daily, as rapid weight loss and gain are associated with changes to fluid balance (Donohoe, 2016; Sabino et al, 2016). Urinary output and other fluid losses may be compared with IVFT administration, nutrition and water intake for a more complete analysis of fluid balance.
Nutrition
Appropriate nutrition is beneficial to the hospitalised patient, such as the uroabdomen patient, as failure to do so results in an impaired immune response and delayed wound healing (Chan, 2016). Provision of nutrition is often the responsibility of the nursing team, who should aim to feed patients 100% of their resting energy requirement to ensure an appropriate calorie intake (Gajanayake, 2020). Starting nutritional support early in hospitalisation has been demonstrated to improve patient outcome, so this should be done as soon as is feasible as agreed by the veterinary surgeon (Tonozzi, 2017). Ideally the patient will eat normally, so nursing care includes tempting feeding with small amounts of food and avoiding food aversion by removing any unwanted diet. If the patient does not eat independently then placement of a feeding tube may be indicated to provide enteral feeding (Ford-Fennah et al, 2020).
Conclusion
A uroabdomen can occur for multiple reasons, with patient prognosis varying depending on several factors: the location of the rupture, the severity of bloodwork derangements, whether the effusion is septic and the occurrence of any surgical or anaesthetic complications (Stafford and Bartges, 2013; Grimes et al, 2020). Recent studies found that 74% of cats and 79% of dogs that presented with a uroabdomen survived to discharge (Grimes et al, 2020; Hornsey et al, 2020). Rapid stabilisation is a crucial step in improving prognosis, however comorbidities may be present, particularly in the trauma patient with concurrent injuries (Stilwell, 2017; Valtolina, 2018). The owner may elect to euthanase if there is a poor prognosis, or other concerns, such as financial constraints or ethical issues.
The uroabdomen patient requires intensive nursing care because of their critical status. Improved RVN knowledge of the pathophysiology of this condition allows for anticipation and identification of any complications, ultimately improving patient welfare.
KEY POINTS
- There are multiple causes of uroabdomen, bladder rupture from trauma is most common in dogs and urethral obstruction is most common in cats.
- Metabolic and electrolyte derangements including hyperkalaemia, azotaemia and hypovolaemia can result in a critical condition.
- Initial stabilisation is essential to improve patient outcome and should include urinary diversion.
- Diagnostic imaging is required to locate the urinary tract rupture, with contrast studies being the preferred method of imaging.
- Surgery may be required depending on the location of the rupture.
- Postoperative nursing care and close monitoring are required for detection of any complications.


