Placement of catheters for venous access provides a standard method to deliver a variety of therapies for hospitalised patients and are commonplace in veterinary care today. Central venous catheters are useful for administering multiple fluids and drugs that are incompatible, high osmolality fluids without causing phlebitis, obtaining frequent blood samples without performing venepuncture each time, delivering parenteral nutrition solutions, measuring central venous pressure, analysing central venous blood saturation, and are used for renal replacement therapy. Contraindications to central line placement include patients at increased risk of thrombosis and hypocoagulation.
Despite their usefulness, placement of venous access catheters is accompanied by the potential of causing local phlebitis and hospital-acquired infections called catheterrelated bloodstream infection (CRBSI). CRBSI is a result of microorganisms being introduced through skin contaminants during placement or contamination of infused fluids or internal surfaces of the intravenous line leading to bacteraemia, and care should be taken during the placement and maintenance of catheters to minimise the chance of its occurrence.
Placement
Many precautions and techniques utilised in the placement of central venous catheters are directed at preventing CRBSI. The common types of central venous catheters are over-the-needle, through-the-needle, peel-away introducer catheters, and guide-wire catheters. Competence and comfort with specific types of catheter is considered on choosing a catheter as it can affect success and degree of contamination. A proper written protocol provides a checklist of key points to follow during placement (Box 1).
Preparation
The coat of the patient over the proposed insertion site is clipped with clean clipper blades taking gentle care to prevent from irritating or abrading the skin while clipping as close to the skin as possible. The area is clipped 3 to 5 cm wide in each direction to prevent contamination from the surrounding fur. The area is then disinfected with a surgical scrub technique consisting of three to seven alternating repetitions of alcohol and an antiseptic soaked gauze in a concentric circular pattern starting at the proposed insertion site. Antiseptics commonly used are chlorhexidine and povidone-iodine. Studies and systematic reviews evaluating whether one antiseptic is superior over the other in prevention of CRBSI summarise results ranging from no difference between chlorhexidine and povidone-iodine to low quality evidence in favour of chlorhexidine (Goudet et al, 2013; Lai et al, 2016). The catheter placement kit and supplies are then opened onto a tray covered with sterile drape material to allow for ample room without risk of contamination of supplies.
Antimicrobial-treated central venous catheters are available and consist of catheters that are impregnated, coated or bonded with antiseptic or antimicrobial compounds. A Cochrane systematic review evaluated the use of 16 784 catheters of 11 different impregnation types and summarised that catheter impregnation with antimicrobials significantly reduces the occurrence of CRBSI in people, although ultimately it did not affect occurrence of sepsis and patient mortality. Thus, routine use of antimicrobialtreated catheters is cautioned since it may contribute to development of resistant organisms and induce allergic reactions (Smarick and Edwards, 2015; Lai et al, 2016).
Catheter placement
Hand washing is performed prior to the placement of the catheter to reduce the amount of potentially pathogenic bacteria on the hands. Hand washing can be performed with soap and water or by using an antiseptic hand wash. An antiseptic alcohol-based rub can substitute for hand washing if there is no fur, blood, dirt or other contaminants on the hands. Sterile gloves are worn during placement through a sterile fenestrated drape over the catheter site (Figure 1), and additional barrier protection such as the use of sterile gown, mask, and cap is recommended as it is observed to reduce CRBSI chances in human medicine (O'Grady et al, 2011).
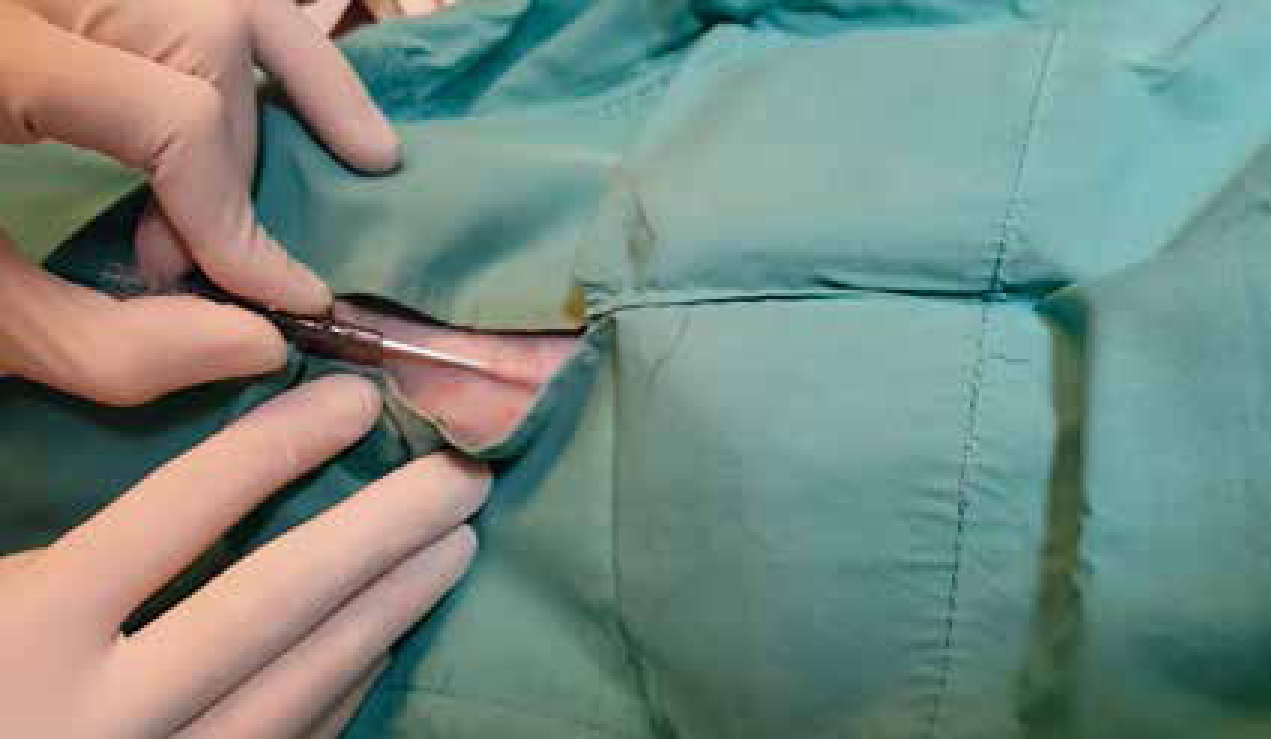
The use of venous cut down procedures or creation of a relief hole in the skin to facilitate the placement of central venous catheters can lead to increased chance of infection as it artificially creates a substantial breach in the skin and is recommended to be minimised (Smarick and Edwards 2015). During the catheter placement care must be taken to prevent any parts of the catheter kit from becoming contaminated. When utilising the Seldinger's (guidewire) technique, one must be mindful of preventing the guidewire from touching outside of the sterile field, and the use of a large drape is recommended. Strict sterile technique is followed during the rest of the placement procedure.
Catheter dressing
The catheter insertion site is covered by sterile non-adhesive gauze or sterile, transparent, semi-permeable dressing to prevent contamination from the patient environment (O'Grady et al, 2011). The relative effectiveness of tape, sterile gauze, sterile transparent dressing, and antimicrobial-impregnated transparent dressing is of interest and limited conclusions can be drawn from investigations. A Cochrane systematic review evaluating the effectiveness of antimicrobial-impregnated dressing used with central venous catheters found that chlorhexidine gluconate-impregnated dressings were more effective in reducing occurrence of CRBSI when compared with standard polyurethane or gauze and tape (Figure 2) (Ullman et al, 2015). The same review also noted that sutureless securement devices were even more effective in reducing chances of CRBSI (Ullman et al, 2015), and the use of these devices in veterinary medicine should be explored as the use of sutures to secure central venous catheters are common and is a potential contributor to hospital acquired infections. The use of topical antibiotic ointment is recommended against because of its potential to promote fungal infections and antimicrobial resistance (O'Grady et al, 2011).
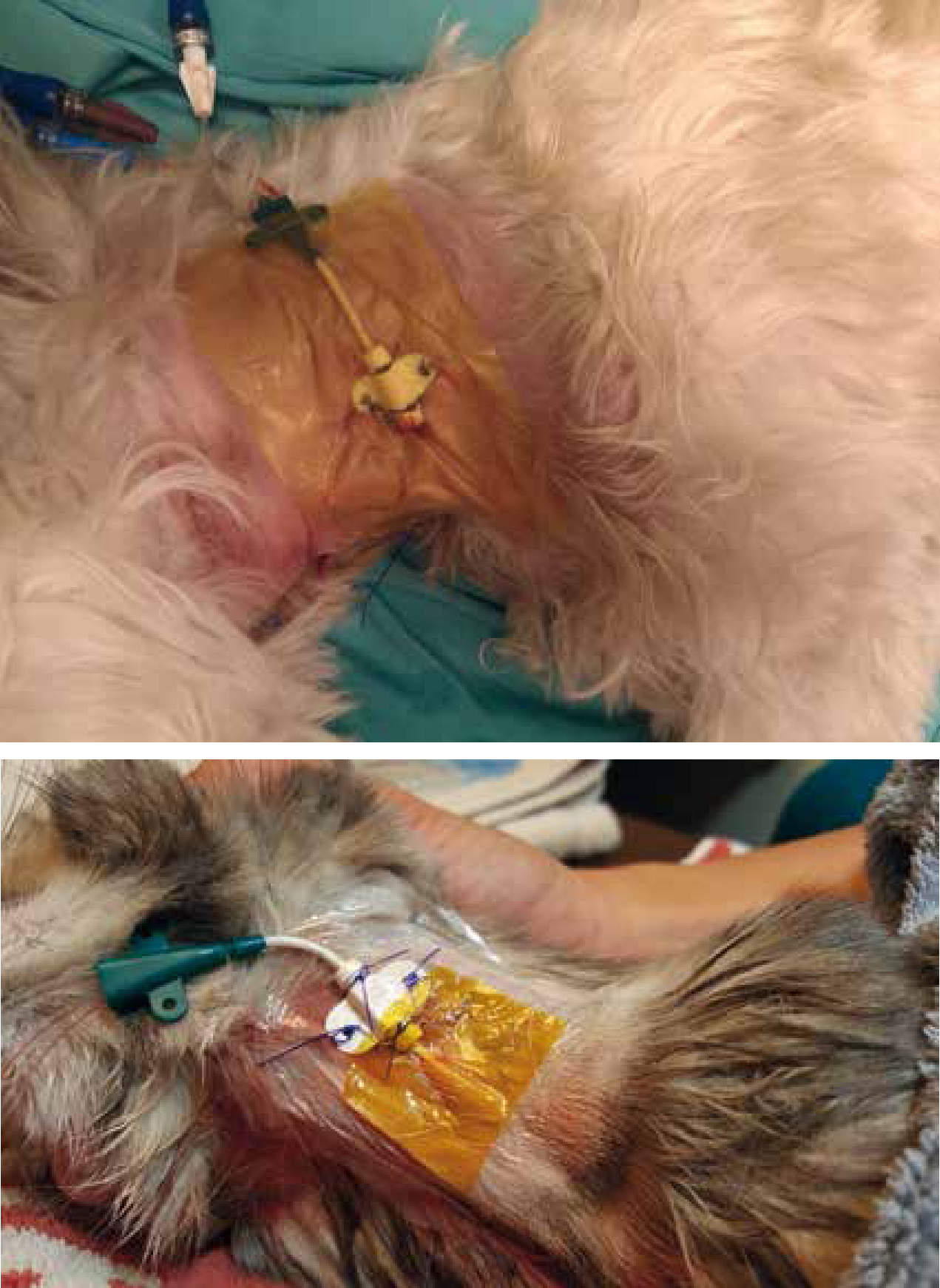
Once the insertion site is covered, a loose bandage should be placed over the neck or extremity in which the catheter is placed by using a combination of cast padding, stretch gauze, or self-adherent cohesive bandage material. The ports can then be taped on the bandage for stabilisation.
Maintenance
Once the catheter has been placed with care to prevent contamination of the catheter and insertion site, a written and well established maintenance protocol will reduce chances of CRBSI. Proper hand hygiene and wearing of clean gloves during the handling of central venous catheters and associated lines are critical to reducing chances of contamination. In addition, scrubbing of access ports in the intravenous lines with antiseptics prior to injections help prevent CRBSI (O'Grady et al, 2011).
Whether heparinised saline should be used instead of normal saline for maintenance of catheter patency continues to be a topic for debate. A veterinary study evaluating the effect of heparinised saline in peripheral catheters with subjective measures saw no statistical difference between catheters flushed with either types of saline (Ueda et al, 2013). Systematic reviews investigating the effect of heparinised saline in maintaining patency in central venous catheters in human adults and infants have both found no conclusive evidence that heparinised saline prevented complications, though still called for additional research to be conducted for a definitive answer (Lopez-Briz et al, 2014; Bradford et al, 2015). Because of a lack of definitive evidence, protocols regarding flushing of central venous catheters vary in the use of heparin.
Dressing checks and changes
The maintenance protocol starts with scheduling of regular visual checks on the dressing of at least once daily. Catheter site dressing should be replaced if it becomes damp, loose, or is visibly soiled. The optimal frequency of dressing change is difficult to determine as systematic reviews conducted revealed the evidence to be inconclusive regarding the difference between longer and shorter intervals in occurrence of CRBSI, mortality, or pain (Gavin et al, 2016). Established guidelines recommend gauze dressing to be changed every 2 days, while transparent dressings can be changed once every 7 days (O'Grady et al, 2011).
Light palpation of the insertion site through the dressing to determine presence of discomfort and visualisation during dressing changes for redness, inflammation, discharge, and phlebitis can allow detection of contaminated insertion sites. If the insertion site shows signs of inflammation or discharge it can be scrubbed with 2% chlorhexidine solutions and dried before reapplying the dressing (Benasutti, 2012). The patient should be evaluated further for signs of CRBSI. Patients developing fevers with no sources aside from the central venous catheter should be suspected of CRBSI and the site more closely evaluated, or removal of the catheter considered (O'Grady et al, 2011; Smarick and Edwards, 2015).
If no abnormalities are seen, a general protocol calls for cleaning of the insertion site at the time of dressing change with 2% chlorhexidine scrub solution and allowing the air to dry or dried with sterile gauze prior to reapplication of new, sterile dressing material (Benasutti, 2012).
Catheter replacement and removal
Routine replacement of the central venous catheter is not recommended and is associated with increased risk of CRBSI (O'Grady et al, 2011). Fever alone does not confirm development of CRBSI in a patient, and clinical evaluation of infection from other sources or non-infectious causes of fever should be considered. Localised infections with signs of purulent discharge and phlebitis can indicate CRBSI, though local signs are not always present. CRBSI is diagnosed through culturing of the blood and catheter as clinical signs alone are not sufficient. Routine screening by qualitative catheter tip or segment cultures is not recommended because of the prevalence of false-positive results (Smarick and Edwards, 2015).
Central venous catheters can be replaced by guidewire exchange, or the reintroduction of the guidewire and removal of the catheter followed by insertion of a new catheter over the guidewire. Guidewire exchange is the recommended method of replacement of non-functional central venous catheters, but is recommended against when CRBSI is suspected (O'Grady et al, 2011). The central venous catheter should be removed as soon as its clinical necessity is alleviated, as the length of dwell time will significantly increase the risk of CRBSI (Benasutti, 2012).
Conclusions
While central venous catheters have established benefits in therapeutic options and monitoring provided, they do not come without a cost. With lengthy hospitalisation periods being a real possibility in a patient requiring critical care, careful consideration and refining of protocols to minimise the chances of hospital acquired infections is a necessary step towards maximising chances of a positive patient outcome. Knowledge and practice in applying the protocol and using proper technique is a veterinary nurse's responsibility.

