The use of acupuncture in modern veterinary practice is becoming increasingly popular in the treatment of both acute and chronic painful conditions. Having evolved over thousands of years, acupuncture has become a scientifically driven, medically appropriate therapy for patients of human and veterinary medicine (Park, 2002). An increasing number of experimental and clinical studies are now showing measurable clinical benefits with specific biological effects following acupuncture treatments (Cheng, 2014; Fry et al, 2014). By inserting a thin sterile needle into a series of acupuncture points, a compressive treatment is created which addresses discomfort and disease via a multitude of path-ways. Acupuncture points have specific anatomic locations, and with advances in neuroimaging and molecular biology techniques, they have been found to be richly innervated by small arterioles and venules, lymphatics, free nerve endings, immunomodulatory cells such as mast cells, and in regions with an autonomic nervous association. Examples include sites of nerve branching, neurovascular bundles, foraminal exits, and nerve penetration of fascia (Ma, 2005; Dewey and Xie, 2021). Many acupuncture points are in close proximity to regions that generate pain and muscular dysfunction, which include musculotendinous junctions, myofascial trigger points, and muscle motor points. The sole targeting of myofascial trigger points with acupuncture needles is a common practice used by both human and veterinary practitioners and is sometimes referred to as ‘dry needling’ (Fry et al, 2014).
Acupuncture points share histological features, which include infiltration of afferent receptors such as nociceptors and Meissner corpuscles (Abraham et al, 2011), nervi vasorum, neuromuscular junctions, and Golgi tendon organs (Ma, 2005). Acupuncture points also contain a high concentration of gap junctions, which may explain the enhanced conductivity of bioelectrical signals that we see in these regions, and is enhanced by nitric oxide (NO). Gap junctions contain a higher concentration of neuronal nitric oxide synthase (nNOS), in addition to transient receptor potential vanilloid type-1 (TRPV-1) receptors; with the expression of both upregulated following an electroacupuncture treatment. These receptors are cation channels, which are an integral part to pain transmission and are prevalent within the C fibers which function in the transmission of pain from the periphery to the central nervous system (CNS) (Fry et al, 2014; Dewey and Xie, 2021).
With the rich diversity of acupuncture point types, a broad treatment using multiple mechanisms can be achieved using a combination of these points to treat a patient's pain condition. Electroacupuncture is a type of acupuncture whereby a gentle electrical current is passed through the needles, and can be applied to several acupuncture points. This is a common adjunct to acupuncture treatments and provides a more vigorous and prolonged stimulation to the needles, thereby enhancing the acupuncture treatment. The frequency at which the electrical current is applied to the needles can be altered to the individual patient and their needs.
Acupuncture is becoming increasingly accepted in both human and veterinary medicine as part of a multimodal approach to the management of acute and chronic pain. Acupuncture's analgesic effects can be conceptually divided into local, segmental (spinal), and suprasegmental (brain) effects (Figure 1) (Fry et al, 2014; Dewey and Xie, 2021). It is a minimally invasive treatment, with very low risks of adverse effects, and is a pleasurable experience for both the patient and client.
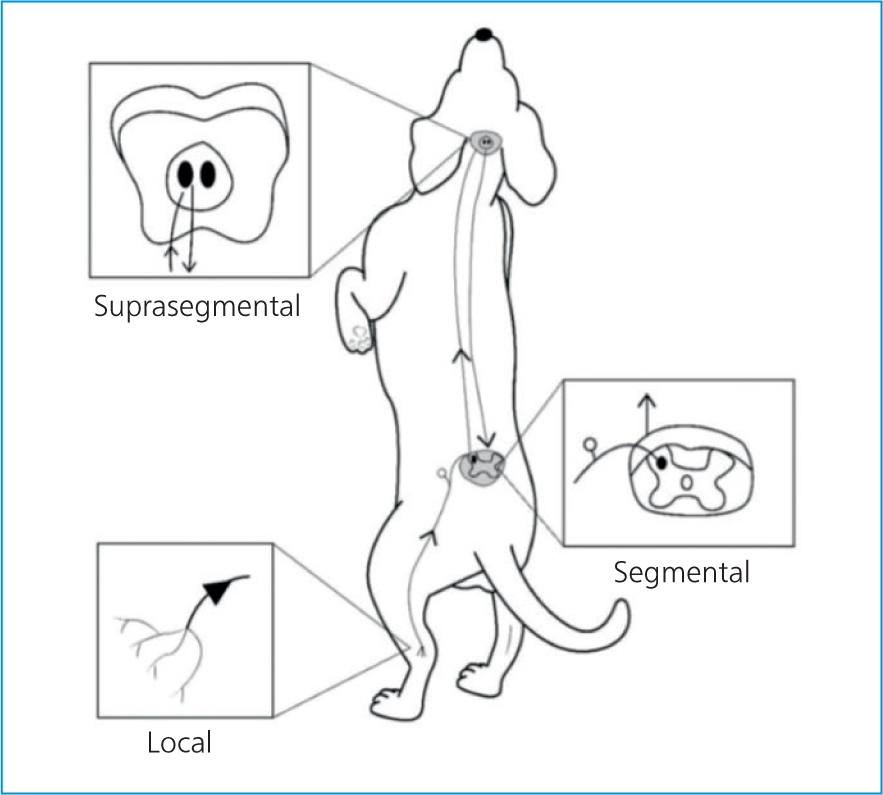
With increased understanding of pain signalling path-ways through advanced neuroimaging and molecular biology techniques, we are starting to gain more of an insight into the intricate mechanisms behind the physiology of acupuncture and its analgesic properties; this paper aims to raise some awareness into how acupuncture can be of benefit to many veterinary patients.
Local effects
The plethora of structures at acupuncture points such as: blood vessels, immunomodulatory cells and afferent nerve endings that respond to touch, pressure, pain, and chemical changes; muscles; glands; and connective tissues, all contribute to its effects. These structures interact with the acupuncture needle near the insertion site and the nervous system is stimulated both locally and distally. Early studies revealed that the analgesic effects of acupuncture could be blocked if the associated area of skin was blocked with local anaesthetic (Chiang, 1973). This emphasised that there is a strong connection between neuronal signalling and needle insertion. After the needle is inserted and manipulated primarily by twisting, there is a coupling between the local connective tissues and the needle. Collagen and elastin fibers wind around the needle which leads to deformation of the local collagen matrix. A ‘pull-out force’ is created by the grab of the local tissues on the needle as the needle is removed from the tissue, which acts on local nerve endings, vasculature, and on cells such as fibroblasts (Langevin et al, 2001). The stimulation of local nerve endings leads to a response that is responsible for a cascade of local cytokine release, which in turn leads to mast cell activation and blood vessel dilation (Zhang et al, 2008). These local effects on both skin and muscle blood flow are intensified with deeper needle insertion and greater manipulation. A variety of neuropeptides, such as nerve growth factor, calcitonin gene-related peptide (CGRP), vasointestinal active peptide, substance P, and many more are released in response to the stimulation of local nerve endings. This release of cytokines and neurotransmitters in addition to the changes in local blood flow have shown to contribute to improve both local healing and local analgesia (Figure 2) (Zhang et al, 2008).
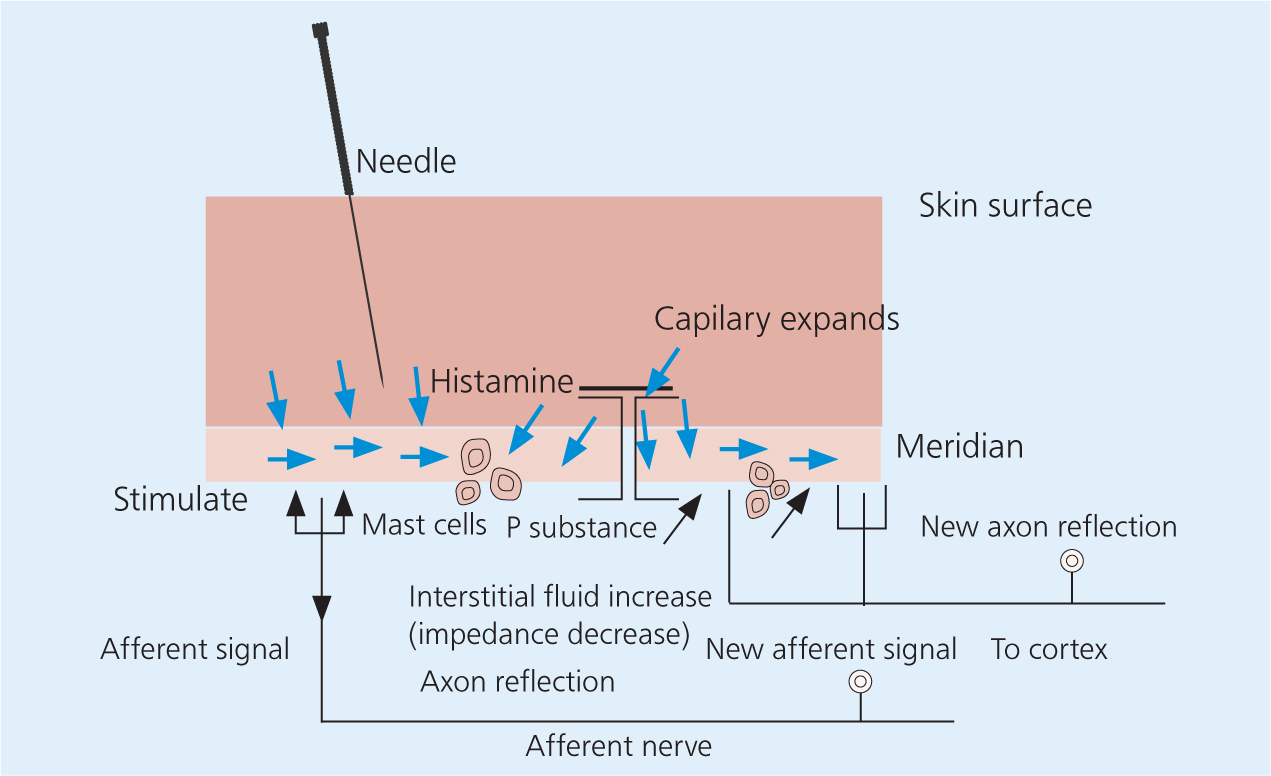
Acupuncture stimulates the local release of endogenous opioids from lymphocytes, macrophages, and granulocytes within the surrounding tissues (McDonald et al, 2015). These opioids act on peripheral nerve receptors within the tissue to suppress the propagation of nociceptive signals (Zhang et al, 2014). An acupuncture treatment can be enhanced by providing electrostimulation of the acupuncture points, which alters the release of opioid peptides and the types of peptides being released. Beta endorphin, encephalin, and orphanin release can be observed following a low-frequency stimulation (2-4 Hz), whereas serotonin and norepinephrine release is observed after higher frequencies (80–100 Hz) (Han, 2003, 2004; Zhang et al. 2014). It has also been found that the analgesic effects lasted longer and were more cumulative after using lower electrical frequencies. Various studies have shown that elevated levels of beta-endorphins contributed to prolonged analgesia of at least 24 hours in dogs treated with electroacupuncture after ovariohysterectomy when compared with butorphanol analgesia alone (Groppetti et al, 2011). It is this induction of endogenous opioids, especially following electroacupuncture that is perhaps the most accepted mechanism of action for acupuncture analgesia (Zhang et al, 2014).
Acupuncture is considered a method of counter-irritation that provides sensory stimulation to the local tissues (Dewey and Xie, 2021). Several anti-inflammatory and immune responses are initiated following needle stimulation. The opioids released work by acting on the receptors of peripheral nerves, thereby suppressing the propagation of nociceptive signals. Opioid levels within the region are further increased with the activation of peripheral sympathetic nerve fibers. Adrenergic receptor activation of inflammatory cells contributes to this effect by releasing β endorphin into the local tissues. Sympathetic nerve fiber activation also contributes to the increased expression of intracellular adhesion molecules within the blood vessels of inflamed tissue. This promotes neutrophil and mononuclear cell migration which contain β endorphin and met-enkephalin (Dewey and Xie, 2021). There is an increase of cannabinoid CB2 receptor expression within tissues following acupuncture, which leads to an upregulation of local opioids. Local inflammatory cytokines including interleukin 1β (IL-1β), tumour necrosis factor-α (TNFα), and interleukin 6 (IL-6) are reduced following an acupuncture treatment (Fry et al, 2014). Acupuncture also acts locally by inhibiting the production of cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2) within the stimulated tissues (Dewey and Xie, 2021). Electroacupuncture has been shown to reduce levels of nerve growth factor (NGF), which is associated with inflammation. There is also an upregulation of TRPV-1 receptors at peripheral nerve endings after acupuncture, and this is associated with an analgesic affect. ATP and adenosine play an important role in pain modulation both peripherally and centrally, and are released by the local subcutaneous mast cells on acupuncture stimulation (Fry et al, 2014; Dewey and Xie, 2021).
Many clinical conditions have been shown to benefit from these local analgesic properties such as: burns and abrasions, surgical incisions, acute and chronic wounds, local dermatitis, lick granulomas, local injuries to the joints, muscles, and other soft tissue injuries (Fry et al, 2014).
Segmental effects
During embryogenesis the spinal cord segments and vertebral column develop in close synchrony, which accounts for the way in which the nerves and roots of each spinal cord segment are distributed among the vertebral column. As the somatic dermatome (area of skin innervated by a single spinal cord segment) expands over the body surface, its original segmental innervation is maintained. There is significant overlap of the peripheral nerves within these cutaneous regions. Because of this connectivity via shared innervations, every part of an individual segment has the potential to modulate other parts of that same segment. This type of segmental innervation is particularly useful when direct needling is inappropriate and an indirect approach to needling is favourable, such as with visceral disease (De Lahunta et al, 2014).
It is not possible to specifically isolate segmental (spinal) and suprasegmental (brain) effects of acupuncture as they are both interconnected anatomically and physiologically. However, acupuncture (in particular EA) induces many antinociceptive effects at the spinal level. There is a reduction in N-methyl-D-aspartate (NMDA) receptor activity in the spinal cord dorsal horn via increasing levels of endogenous opioids, noradrenaline, and serotonin. The NMDA receptor is activated in the modulation of chronic and pathologic pain states leading to spinal cord sensitisation and hyperalgesia. This activation leads to a greater, more painful response to stimuli by altering the threshold to nerve firing. This is a major component of hyperalgesia and allodynia (Zhao, 2008). Through its effects on reducing NMDA receptor activity and reduction in hyperalgesic intensity, acupuncture offers great potential in the treatment of chronic pain states (Fry et al, 2014).
The increase in serotonin levels within the spinal cord helps accentuate the ability of gamma aminobutyric acid (GABA) in its inhibition of pain signal transmission (Fry et al, 2014; Zhang et al, 2014; Dewey and Xie, 2021). The activation of spinal glial cells is reduced by acupuncture, which leads to a reduction in the production of pain promoting cytokines (IL-1β, IL-6, TNF α, COX-2, and PGE2) from astrocytes and microglial cells. The release of substance P in the spinal cord grey matter is also inhibited by acupuncture. Nociceptive signals and glial cell activation are promoted by substance P. Electroacupuncture also stimulates the release of GABA from the synapses between dorsal root ganglion neurons and the spinal dorsal horn neurons. Nociceptin/orphanin FQ (N/OFQ) receptors have been shown to be upregulated within the spinal cord grey matter following acupuncture. N/OFQ is an opioid-like peptide with potent analgesic properties and is widely dispersed throughout the spinal cord. It works by inhibiting C fiber-evoked responses and wind up. Acetylcholine and dopamine levels at the level of the spinal cord dorsal horn have been shown to increase after an acupuncture treatment and is believed to have an antinociceptive effect. Similar to its action on the periphery, acupuncture blocks the expression of COX-2 at the level of the spinal cord. In contrast to the peripheral nerve endings, TRPV-1 expression has been shown to be downregulated in the spinal cord dorsal horn neurons (Dewey and Xie, 2021).
When dealing with patients with osteoarthritis, pain relief is paramount owing to its association with increased morbidity and mobility loss (Figures 3 and 4). Segmental analgesia in conjunction with pain relief may help to reverse pathological loss of muscle tone, and encourage movements, which in turn promotes blood flow and improves healing (De Lahunta et al, 2014). Weight bearing behaviour was improved and articular afferent neural responses to noxious stimulation was inhibited in an animal model of arthritic stifle pain (Oh et al, 2006). Similar results were found in a study that included nearly 4000 human patients (Cao et al, 2012).


When electroacupuncture was used in combination with standard Western medical management in dogs with signs of thoracolumbar intervertebral disc disease (IVDD), a shorter time to deep pain perception and ambulation was observed than in cases managed medically alone (Hayashi et al, 2007). Acupuncture has been shown to be useful in the conservative orthopaedic management of chronic lower back pain in humans, with an improvement seen to last 6 months or longer (Molsberger et al, 2002; Haake et al, 2007).
Segmentally, the somatic and visceral afferent information converges at the dorsal horn. ‘Referred pain’ that may originate from an abdominal organ can be perceived as arising from the abdominal wall musculature, which is supplied by the same segmental innervations. Visceral pain may be suppressed by reversing this process through the stimulation of appropriate somatic receptors at the same segmental level, which is most useful if it is not possible to use local acupuncture points. Visceral nociception of ‘referred pain’ has a dermatomal distribution and the surface of the body has been well mapped in humans to represent the areas of pain associated with the various visceral organs. These regions are not as well localised in animals, however, clinical examination of animals with known visceral pain often show a consistency in areas of cutaneous sensitivity similar to those found in people. In addition to pain relief, acupuncture also alters organ blood flow and the autonomic nervous system which regulates visceral function. This has been shown through the effective use of electroacupuncture in the treatment of visceral pain associated with irritable bowel syndrome in a rat model (Cui et al, 2005). The use of acupuncture at the level of visceral autonomic innervation can normalise the autonomic output and reduce pain. Segmental acupuncture analgesia may be beneficial to numerous conditions such as: neuropathies, IVDD, painful visceral conditions, and joint dysfunction — particularly in those as a result of osteoarthritis (Fry et al, 2014).
Suprasegmental effects
Acupuncture also derives some of its analgesic effects from changes within the CNS. The suprasegmental effects of acupuncture are believed to be attributed to the stimulation of a descending antinociceptive network which projects from several regions of the brain to the spinal cord dorsal horn (Fry et al, 2014; Dewey and Xie, 2021). The brain regions involved include the hypothalamus, the dorsolateral prefrontal cerebral cortex and cingulate gyrus, the ventral tegmental area of the midbrain and the periaqueductal gray (PAG) matter, the raphe magnus nucleus of the medulla, and the locus ceruleus of the pons (Zhao, 2008). These various regions of the brain either indirectly, or directly suppress nociceptive spinal cord dorsal horn neurons via several different cytokines such as enkephalins, β-endorphins, dopamine, noradrenaline, and serotonin. The pituitary gland also functions within this network and releases oxytocin and adrenocorticotrophic hormone into the blood stream following acupuncture stimulation, which has an analgesic effect (McDonald et al, 2015). Specific acupuncture points, when stimulated, have been shown to activate specific brain regions from positron emission tomography studies and functional magnetic resonance imaging studies in people. Some examples of suprasegmental acupuncture effects (especially electroacupuncture) include activation of noradrenergic neurons within the locus ceruleus of the pons, activation of serotonergic nucleus raphe magnus (NRM), neurons in the medulla, hypothalamic stimulation to release β-endorphin, and descending neuron activation of the mesencephalon PAG matter (Dewey and Xie, 2021). Acupuncture has variable effects on TRPV-1 expression in the brain and appears to be location dependent. This has been shown in a rodent study where electroacupuncture was used for the alleviation of inflammatory pain. TRPV-1 levels in the prefontal cortex and hypothalamus were reduced after a treatment but were potentiated in the PAG matter of the midbrain (Dewey and Xie, 2021).
It is suggested through imaging studies that the PAG, although working in concert with other midbrain structures, is the primary CNS structure for the inhibition of descending pain (Fry et al, 2014). It is well known that acupuncture works via noradrenaline and serotonin release at all dorsal horn segments and therefore overlaps with the segmental analgesic effects of acupuncture and reinforces both local and segmental analgesia. The central mechanisms of acupuncture analgesia could be beneficial to many conditions including: anxiety, stress, and chronic pain especially from orthopaedic and neurologic conditions (Fry et al, 2014).
Myofascial trigger points and acupuncture
Myofascial trigger points are defined as hyperirritable areas usually present within a taut band of skeletal muscle which is painful on compression giving rise to tenderness, pain, motor dysfunction and autonomic phenomena (Simons, 1999). It is now widely accepted that trigger points are an important source of pain in both human and veterinary patients. These trigger points are a result of chronic muscle overuse, altered posture, acute overload, metabolic disease, and traumatic injury. By inserting acupuncture needles directly into trigger points there can be an immediate reduction in pain and an improvement in some of the associated underlying pathophysiological changes can be observed. When directly palpated, trigger points may have an associated twitch response. It is important to achieve a local twitch response with needle insertion to accomplish immediate pain relief for the patient (Tekin et al, 2013). Rapid twisting and redirection of the needle is usually well tolerated by the patient and can be helpful in achieving the local twitch response required. Although the mechanism for trigger point release is not well defined, an immediate reduction in regional pain has been shown that is likely through alterations in dorsal horn modulation of trigger point pain (Srbely et al, 2010). Additionally, acupuncture also inhibits spontaneous motor endplate noise at both local and distal sites (Chou et al, 2009). This treatment of myofascial trigger points can be of benefit to any patient with primary musculoskeletal injuries, or others such as neurological injuries that alter muscular mechanics or tension.
A general sense of wellbeing and analgesia at sites distant to the acupuncture needle has often been reported in human patients. This is also believed to be perceived by veterinary patients. It is likely that needle placement brings about the modulation of neurotransmitters and other neuromodulatroy cytokines leading to a sense of generalised analgesia (Fry et al, 2014).
Examples of conditions that could benefit from trigger point acupuncture include: orthopaedic injury and surgery, spinal cord injury, peripheral nerve injury, head tilt or torticollis, and osteoarthritis particularly in those patients with an altered posture or gait.
Other conditions that may benefit from acupuncture
The physiological pathways for ‘non-pain’ conditions are less well mapped, however, good results have been seen in practice for many conditions, such as interdigital granuloma in the dog; a reduction in the straining and pain associated with urethral obstruction in the cat; sweet itch in horses among many others.
Clinically, gastrointestinal disease has been shown to respond to acupuncture treatments. The mechanisms still remain unclear, but it is hypothesised that the somatic stimulation from an acupuncture treatment has a normalising effect on gastrointestinal tract autonomic tone through its effects on the innervating sympathetic and parasympathetic nerve fibers that originate from the spinal cord (Yin and Chen, 2010).
In dermatology, acupuncture has the potential to aid in the treatment of atopic dermatitis through its antipruritic effects; however the evidence base for this remains unknown. Studies exist that suggest that acupuncture has a role in itch reduction and regulating IgE-mediated allergy, which are major components of atopic dermatitis. As no randomised controlled trials (RCTs) exist for these studies, there is a great need for the rigorous design of RCTs to assess acupuncture's role and efficacy in the management of atopic dermatitis (Tan et al, 2015).
Conclusion
Acupuncture offers an effective, well-documented analgesic approach to the management of pain in veterinary patients. Through advances in molecular biology techniques, numerous mechanisms have been discovered that explain the pain-mitigating effects of acupuncture. With improved understanding of the physiological mechanisms of acupuncture analgesia it will continue to grow in popularity within veterinary practice. When performed by a trained professional, it is a safe treatment with very few reported side effects, and should be considered as a powerful adjunctive tool in the multimodal analgesic approach to many of the painful conditions encountered on a daily basis within the veterinary practice.
KEY POINTS
- Acupuncture is becoming increasingly popular within both human and veterinary medicine as a useful adjunct to the analgesic treatment of many painful conditions.
- With the connections between the peripheral and central nervous systems, multiple regions within the body can be accessed through individual acupuncture points.
- The analgesic effects of acupuncture can be divided into local, segmental (spinal), and suprasegmental (brain) effects based on which antinociceptive pathways become activated by needle stimulation.
- A more potent response to an acupuncture treatment can be achieved by passing an electrical current through the acupuncture needle (electroacupuncture), which in turn leads to greater substance release.
- Clinically, acupuncture has shown to be effective when used adjunctively to treat other ‘non-pain’ conditions such as gastrointestinal disease and atopic dermatitis, among others.


