References
Biofilms and their significance in veterinary wound management
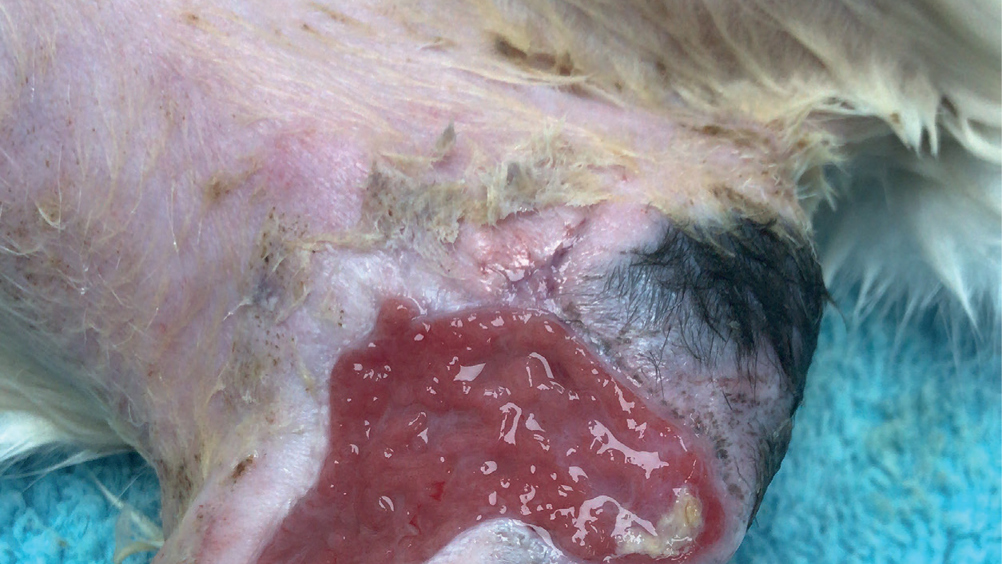
Abstract
Wound management in veterinary practice can be challenging at times. With a variety of factors to consider when managing a wound, it can be further complicated if that wound starts to stagnate or regress. A process of elimination begins to identify the cause of the problem. Infection is a contributing factor to delays in wound healing, but biofilms may not always be considered, especially during the initial stages. These communities of bacteria need to be managed quickly and efficiently to prevent them becoming irreversibly embedded within a wound. As nurses it can be a useful wound management skill to be able to recognise biofilms and their signs and understand how early interventions and appropriate wound management can prevent a biofilm from becoming a problem.
Chronic wounds can present a variety of challenges in veterinary practice, and it can become time consuming and expensive when trying to eliminate what exactly may be causing a delay in wound healing. A chronic wound can be defined as a wound that has failed to progress through the natural stages of healing and remains in a continual and unmanageable inflammatory phase despite wound management interventions (Zhao et al, 2016). When presented with a wound that had been previously granulating, but seems to be regressing or showing signs of a prolonged inflammatory response, it is important to consider the reasons that this could be happening. Using the basic principles of wound management and considering the factors that contribute to delays in wound healing may help to highlight what might be contributing to the stagnation or prolonged inflammatory response within a wound bed. Presence of infection, movement, patient interference, ischaemia and tension are a few factors that can contribute to a delay in wound healing. Some of these can be eliminated more easily than others, such as patient interference, but when it comes to factors like infection, eliminating certain types of bacteria especially those found in biofilms can prove difficult.
Register now to continue reading
Thank you for visiting The Veterinary Nurse and reading some of our peer-reviewed content for veterinary professionals. To continue reading this article, please register today.

