Blood tests are commonly performed in the intensive care unit (ICU), providing a considerable amount of information about veterinary patients. In the emergency and critical care (ECC) setting, initial blood tests are routinely carried out as part of the secondary survey to provide essential knowledge of a patient’s current clinical status, facilitating the veterinarian’s decision for further diagnostics and a plan for stabilization and treatment. Following admission, blood tests enable the veterinary nurse to monitor the patient’s progress and tailor treatment accordingly.
The role of the veterinary nurse is not only to run these tests promptly and accurately, but in the busy ICU nurses are often relied on to differentiate between those results that should be brought to the immediate attention of the attending veterinarian and those that can wait until the end of the next consult (Randles, 2005).
This article provides an overview of the most commonly screened parameters in the ECC, and an insight into the abnormal values that may pose a life-threatening risk to the patient if left untreated, which require immediate action by the veterinary nurse.
Packed cell volume
Packed cell volume (PCV) is used to determine the red blood cell mass as a percentage of blood volume and should always be interpreted in combination with a total protein (TP) measurement to minimize errors in interpretation and diagnosis (Davis, 2001).
Following the first baseline sample, subsequent tests should be performed at appropriately staged intervals (minimum of once per 24 hours) in order to assess for any ongoing blood loss and the patient’s response to fluid therapy. As well as any initial abnormalities, it is important to draw the veterinarian’s attention to any changes in trends of the PCV, such as a sudden drop or increase over a short time period or a gradual but consistent decrease or increase over a more prolonged period, which can indicate underlying pathology (Table 1).
| Packed cell volume | Total protein | Interpretation |
|---|---|---|
| Increased | Increased | Dehydration |
| Increased | Normal or decreased | Splenic contraction (polycythaemia or hypoproteinaemia) |
| Normal | Increased | Normal hydration with hyperproteinaemia, anaemia and dehydration |
| Decreased | Increased | Anaemia and dehydration or anaemia with hyperproteinaemia |
| Decreased | Normal | Non-blood loss anaemia with normal hydration |
| Normal | Normal | Normal, acute haemorrhage, dehydration and anaemia and hypoproteinaemia |
| Decreased | Decreased | Blood loss, anaemia and hypoproteinaemia or overhydration |
Total protein
TP measures the albumin, globulins and fibrinogen in plasma, which contribute to the colloid osmotic pressure (COP). COP is the osmotic force created by large molecules, preventing the movement of water out of the vessel and into the interstitial space (Rudloff and Rivera, 2012). TP in combination with PCV can assist in the determination of certain disease processes, such as oedema, ascites, infections, coagu-lopathies, diarrhoea, weight loss and renal or hepatic disease (Table 1).
When performing a TP measurement it is important to assess the serum colour following centrifugation (Figure 1). Normal serum should appear straw coloured and clear; however, the following appearances should be brought to the attention of the veterinarian:

As with PCV, any sudden or consistent changes should be brought to the attention of the veterinarian.
Glucose
Glucose is an essential source of energy for all cells and is regulated in the body by insulin and glucagon. Hypoglycaemia is the primary concern in the ECC setting, and can be caused by excess insulin production (either iatrogenic or endogenous), increased glucose utilization (such as a result of increased exercise levels or endotoxae-mia), decreased glucose production (as a result of hepatic disorders or starvation) or cortisol deficiency. Clinical signs include weakness, altered mentation, ataxia, seizures and coma (seen at <2.2 mmol/l), and treatment should be administered immediately (Wunn, 2009).
Hyperglycaemia can be caused by increased glucose production (as a result of stress, excitement or post prandially) or diabetes mellitus (as a result of insulin defiance or insulin resistance). Hyperglycaemia is generally less critical than hy-poglycaemia, although plasma glucose in excess of 55 mmol/l can lead to altered mentation or coma as a result of the induced hyperosmolar state; thus it is important to instigate prompt treatment (Wunn, 2009).
Close monitoring of these patients should be carried out at regular intervals following treatment (sampling every 1–2 hours initially) to observe their response, and treatment altered as necessary.
Electrolytes
Maintaining the correct balance between electrolytes in the body is essential to ensure normal cell function and intercompartmental water balance (Davis, 2001). Changes in electrolyte concentrations can originate from a number of disease processes, and as a result animals in the ECC setting often have electrolyte imbalances on presentation. It is important to re-check these parameters regularly to monitor response to treatment and to ensure that further imbalances do not occur as a result of treatment. The main electrolytes that are monitored are highlighted below; Table 2 outlines the normal reference ranges for blood parameters in dogs and cats.
| Canine | Feline | |
|---|---|---|
| PCV (%) | 37–55 | 25–45 |
| TP (g/l) | 54–71 | 60–86 |
| Glucose (mmol/l) | 3.6–6.2 | 3.7–9.3 |
| Sodium (mEq/l) | 140–150 | 146–157 |
| Chloride (mEq/l) | 109–120 | 116–126 |
| Potassium (mEq/l) | 3.9–4.9 | 3.8–4.8 |
| pH | 7.41 (7.35–7.46) | 7.39 (7.31–7.46) |
| PaCO2 (mmHg) | 37 (32–43) | 31 (26–36) |
| HCO3 (mEq/l) | 22 (18–26) | 18 (14–22) |
| SBE (mEq/l) | –2 (–5 to +1) | –5 (–2 to +8) |
| Pa02 (mmHg) | 92 (81–103) | 107 (95–115) |
| Lactate (mmol/l) | 0.5–2.0 | 0.5–2.0 |
| ACT (s) | 60–125 | 60–125 |
| PT (s) | 6.8–10.2 | 9.6–13.2 |
| aPTT (s) | 10.7–16.4 | 12.6–15.7 |
From Silverstein and Hopper (2009) ACT, activated clotting time; aPTT, activated partial thromboplastin time; HCO3, bicarbonate; PaCo2, partial pressure of carbon dioxide; Pa02, partial pressure of oxygen; PCV, packed cell volume; PT, prothrombin time; SBE, standard base excess; TP, total protein
Sodium (Na+)
Sodium and water levels are tightly controlled through renal excretion and conservation in order to maintain the volume and tonicity of body fluids within a narrow normal range (Di Bartola, 2006a).
Hypernatraemia is caused by a loss of hypotonic fluid, inadequate water intake or ingestion of high-sodium substances (Rahilly, 2012). Clinical signs are dependent on the rate of change as well as the absolute value (not usually seen until sodium levels are in excess of 170 mmol/l), and include anorexia, polydip-sia, nausea, weakness, ataxia, depression, collapse, coma and death.
Hyponatraemia is caused by excessive water intake, inadequate reabsorption of sodium by the kidneys or disproportionate reabsorption of water to sodium by the kidneys. Clinical signs are not usually seen until the concentration drops to below 125 mmol/l, and include vomiting, ataxia, depression, weakness, seizures, coma, hypotension and shock as a result of cerebral oedema (DiBartola, 2006a).
Identification and treatment of sodium imbalances require close monitoring by the veterinary nurse as it is essential that the concentration is not adjusted too rapidly. Rapid adjustment by >0.5–1.0 mEq/l/ hour or >10 mEq/l/24 hours can lead to neurologic sequelae; thus sodium levels should be checked every 2–4 hours over the initial phase of treatment (Rahilly, 2012).
Chloride (Cl–)
Chloride helps to maintain extracellular osmotic pressure and regulate water balance, and as a result changes in concentration are often, but not exclusively, aligned with changes in sodium concentration.
Hyperchloraemia can be seen with diarrhoea and renal disease. Hypochloraemia can be seen with vomiting, gastrointestinal obstruction and the use of some diuretics, such as frusemide, which block renal absorption of chloride.
Potassium (K+)
Potassium is the principle intracellular cation and is responsible for excitability and function of nerve and muscle cells (Riordan and Schaer, 2009). The main concern when measuring serum potassium levels is to detect changes in concentration, which may cause cardiac toxicity.
At levels >7.5 mEq/l, hyperkalaemia can be life threatening because of the effect of increased potassium on cardiac cell function; it is therefore essential that these results are brought to the attention of the veterinarian immediately (Riordan and Schaer, 2009). Treatment includes intravenous fluid therapy and addressing the underlying cause where possible (i.e. relieving urinary obstruction). Depending on the extent of hyperkalaemia, calcium gluconate or chloride may be administered to protect the cardiac cells, and in severe cases insulin and glucose may be administered to promote the intracellular movement of potassium.
At levels <2.5 mEq/l, clinical signs of hypokalae-mia may be seen, including weakness, cervical ven-troflexion, inability to concentrate urine, vomiting and anorexia. Treatment involves potassium supplementation, which should be administered at low rates (≤0.5 mEq/kg/hour) to avoid adverse cardiac effects, and the patient should be closely monitored (DiBartola and Autran de Morais, 2006).
Blood gases
Blood gas analysis provides information on the oxy-genation, ventilation and acid–base status of the patient (Table 2). It enables the veterinary nurse to identify the presence and magnitude of an acid–base disturbance as well as establishing its respiratory or metabolic origin.
When looking at oxygenation, arterial samples should be taken as these most accurately represent the oxygenation of blood (Di Bartola, 2006b). However, assuming normal perfusion the correlation between venous and arterial samples is generally considered acceptable for all other parameters (Battaglia, 2001).
pH
Most cellular enzymes can only function within a narrow range of pH; therefore, the acidity of blood is extremely tightly regulated by the body within a range of 7.35–7.45. There are three main processes by which the body achieves this:
It is important to remember that the pH simply tells you the overall acid–base status of the patient; a pH <7.35 reflects acidaemia and a pH >7.45 refects alka-laemia. In contrast, the underlying physiological process that causes the acid–base disturbance is referred to as an acidosis or alkalosis; for example, it is possible to have a patient with a chronic respiratory acidosis who is not acidaemic because of effective metabolic compensation. Metabolic acidosis is by far the most commonly seen acid–base disturbance in the ECC setting; however, any acid–base abnormalities should be brought to the immediate attention of the veterinarian. Table 3 outlines possible causes of primary respiratory and metabolic acidosis and alkalosis.
| Metabolic | Respiratory | |
|---|---|---|
| Acidosis | Lactic acidosis
|
Hypoventilation caused by:
|
| Alkalosis | Vomiting
|
Hypoventilation caused by:
|
CNS, central nervous system
PaC02
PaCO2 is a measure of the partial pressure of carbon dioxide (CO2) in arterial blood and a direct refection of minute alveolar ventilation, which is altered by the respiratory centre of the brain in response to changes in carbon dioxide, oxygen and pH. Decreased or increased PaCO2 refects hyper- or hypo-ventilation respectively, but on its own cannot differentiate between a primary respiratory acidosis or alkalosis and respiratory compensation secondary to a metabolic disorder (Kovacic, 2009).
Where significant hypercapnia is seen despite therapy (PaCO2 >60 mmHg), mechanical ventilation is indicated because of the likelihood of induced hypoxaemia. In hypocapnia, interventions should be taken to suppress hyperventilation when PCO2 <20 mmHg as cerebral vasoconstriction may occur (Kovacic, 2009).
Bicarbonate
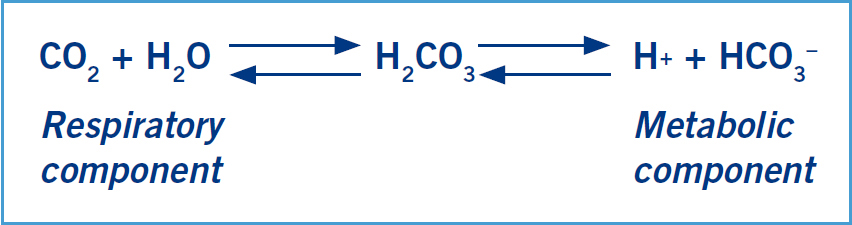
Bicarbonate (HCO3–) is the primary buffer system of the extracellular fluid; it binds to excess hydrogen ions becoming H2CO3 (carbonic acid), which then dissociates to carbon dioxide and water (Figure 2). Carbon dioxide and water are then removed from the body via the lungs and kidneys respectively, and HCO3– is reabsorbed or excreted by the kidneys as required (DiBartola, 2006b). A reduction in HCO3– indicates the presence of a metabolic acidosis, and an increase indicates metabolic alka-losis. Bicarbonate levels can also alter in response to changes in PaCO2; thus it is useful to interpret these results alongside the base excess in order to establish the presence of a primary metabolic cause (Haskins, 2012).
Standard base excess
Standard base excess (SBE) measures the quantity of acid or base required to return the pH to normal at a standard PaCO2 of 40 mmHg (Kovacic, 2009). As it measures values that are not affected by PaCO2, such as respiratory influences, it is considered a more reliable indicator of primary metabolic disturbances. An elevated SBE indicates a primary metabolic alkalosis; a decreased SBE (also referred to as a base deficit) indicates a primary metabolic acidosis (Haskins, 2012).
Pa02
Pa02 measures the partial pressure of oxygen in arterial blood, which is the driving force to push dissolved oxygen into haemoglobin molecules (Irizarry and Reiss, 2009). It is an important monitoring tool for patients with respiratory disorders and gives a clear indication of the need for interventional therapy. A patient with a Pa02 <80 mmHg is considered to be hypoxaemic and in need of oxygen supplementation; if the Pa02 is <60 mmHg despite oxygen therapy, the patient is considered to be severely hypoxaemic and in need of mechanical ventilation (Hopper, 2009). If venous samples are being used for blood gas analysis, a venous PO2 of <30 mm/Hg may indicate poor tissue circulation and should be brought to the attention of the veterinarian (Sorrell-Rashi, 2009).
Lactate
Lactic acid is produced as a result of anaerobic glucose metabolism, which occurs under normal conditions following exercise and other pathological processes. It is converted to lactate and hydrogen ions and controlled at low volumes by the body’s buffering system (Savigny, 2006). The most common clinically significant cause of hyperlacta-taemia (>2.5 mmo/l) is tissue hypoperfusion and subsequent tissue hypoxia; in the emergency setting this is often seen on a systemic level as a result of shock, or on a more localized level such as in a torsion causing localized ischaemia. Immediate fluid therapy to restore effective circulating volume should be instigated alongside establishment of the underlying cause.
Studies have shown that while higher lactate levels correlate with an increase in severity of the underlying cause, it is the failure of a significantly elevated blood lactate level to respond to appropriate initial fluid therapy that is associated with a poor prognosis (Roznaski and Rush, 2007). Seizures, stress, excessive trembling, resisting restraint, excitement and exercise may all cause a mild increase in blood lactate levels within 2 hours of sample collection (Lagutchik, 2009).
Coagulation times
Elevated coagulation times should be brought to the immediate attention of the veterinarian as even stable patients have the potential to decompensate rapidly without treatment (Hackner, 2009). Patients may present with visible active haemorrhage or clinical signs of internal haemorrhage such as anaemia or hypovolae-mic shock; PCV and TP may be normal or reduced.
The three common measures of coagulation testing in the clinical setting are:
Conclusion
The ability of the veterinary nurse to identify critical parameters that require immediate intervention is crucial to optimizing patient care in the ICU. A knowledge of both the normal ranges (Table 2) and the values that can pose a life-threatening risk enables the veterinary nurse to bring them to the veterinarian’s attention promptly. Continued and consistent monitoring of these parameters throughout treatment also facilitates early detection of any deterioration in a patient’s condition, enabling early initiation or alteration of treatment.
Although it is the veterinarian’s responsibility to diagnose patients, veterinary nurses with a sound knowledge and understanding of normal ranges and critical values of blood parameters will be better placed to contribute to the smooth running of the ICU and be able to provide excellent nursing care.
