Urinary tract infections (UTIs) occur when an infectious agent adheres, multiplies and persists within the normally sterile urogenital system. Canine UTIs affect 14% of dogs during their lifetime (Ling, 1984) with 0.3% experiencing recurrent and persistent clinical signs (Norris et al, 2000).
Aetiology
Canine UTIs may occur as a primary disease or secondary to underlying conditions such as diabetes mellitus, hyperadrenocorticism or urinary retention (Forrester et al, 1999; Bubenik and Hosgood, 2008). Decreased host defence mechanisms and increased bacterial virulence are required for the establishment of a UTI (Lulich and Osborne, 2004). Seguin et al (2003) found 71% of dogs with a persistent or recurrent UTI had a primary disorder affecting normal host defences. The majority of UTIs involve bacterial inflammation of the lower urinary tract however potential complications include: struvite urolith formation; acute and chronic prostatitis in entire male dogs; pyelonephritis; renal failure; infertility; lumbosacral discospondylitis; and rarely, septicaemia.
There are three main routes of infection: ascending bacteria; iatrogenic; and haemtogenous.
Escherichia coli is the most commonly isolated bacterial species causing 53.9% of canine UTIs and more often affects female dogs, older dogs and neutered dogs (Hall et al, 2013). Hall et al (2013) looked at 1037 positive urine cultures; 85% contained a single bacterium species, 12.4% had two species, 2.1% three species and 0.3% four species.
Fungal UTIs are rare in dogs with Candida spp. the most common to be cultured (Ling et al. 2001). No viral canine UTIs have been identified.
Host defence
Healthy animals do not routinely develop UTIs due to a number of defence mechanisms (Table 1).
| Defence mechanism | How it works | Conditions predisposing to UTI |
|---|---|---|
| Normal urination |
Complete voiding of the bladder removes >95% of non-adherent bacteria that have ascended the urethra (Nelson and Couto, 2003) | Neurogenic bladder |
| Anatomical structures |
Creates a physical barrier between the bladder and bacteria of the distal urethra | Urinary incontinence |
| Antimicrobial urine |
Urine is frequently bacteriostatic and can be bactericidal | Chronic renal failure |
| Mucosal defence barrier |
Commensal bacteria of the distal urethra compete with pathogenic species. Glycosaminoglycans (GAGs) coat the urothelium protecting against bacterial adhesion. This layer thickens in response to infection | Immunosuppression |
Predisposing conditions
In experimental animals inoculation of the bladder with pathogenic bacteria seldom causes an established UTI unless host defences have been compromised (Rosen et al, 2008). Animals with underlying disease affecting any of these normal defences are vulnerable to infection. Forrester et al (1999) found UTIs occurred in 42% of dogs with hyperadrenocorticism and diabetes mellitus. Clinical signs of UTI were present in <5% of dogs with a positive culture. UTIs are a common and potentially serious complication in dogs recovering from surgery for thoracolumbar intervertebral disc extrusion. Olby et al (2010) found an occurrence rate of UTI in these patients to be 38%, yet 60% of these affected animals had occult disease, i.e. they did not display any clinical signs.
It is therefore recommended that urine culture should be part of any routine diagnostic work up of animals presenting to the practice with UTI predisposing conditions regardless of clinical signs and urinalysis findings.
History and clinical examination
Dogs with UTIs often have an unremarkable physical examination and may or may not have historical clinical signs (Table 2). Lower urinary tract disease is not usually associated with systemic signs; a small, painful and thickened bladder may be found on clinical examination. Pyelonephritis is associated with a history of systemic signs, lumbar pain and a depressed state. A full history and clinical examination is required to look for evidence of an underlying condition which may have predisposed the patient to a UTI. Urinalysis should be caried out in all animals presenting to the practice with UTI predisposing conditions.
| Lower UTI signs | |
| Dysuria | Difficulty urinating |
| Pollakiuria | Increased frequency of urination |
| Stranguria | Straining to urinate |
| Periuria | Inappropriate urination — apparent loss of house training |
| Upper and lower UTI signs | |
| Haematuria | Blood in urine |
| Altered urine | Cloudy appearance, altered odour |
| Upper UTI clinical signs | |
| Polyuria/polydipsia | Increased volume of urine/increased drinking |
| Pyrexia | Raised body temperature |
| Lethargy | |
| Anorexia | |
Urinalysis
Urine collection
There are three main routes of urine collection (Table 3). A complete routine urinalysis including specific gravity, dipstick and sediment examination should be performed ideally within 30 minutes of collection.
| Collection method | Pros | Cons |
|---|---|---|
| Free catch |
Inexpensive |
Unsuitable for culture due to high levels of contamination |
| Catheterisation | Quantitative interpretation of culture results is useful | Potential damage to urethra or urinary bladder |
| Cystocentesis | Gold standard method for urine culture | May require ultrasound guidance in larger animals |
Gross appearance
Observe the urine sample in a transparent container making a note of turbidity (cloudiness), colour and smell. Normal urine should be clear and yellow to straw coloured.
Specific gravity
Specific gravity is easily measured and should be noted for every urinary sample taken. A specially calibrated refractometer should be used to measure specific gravity. Some dipsticks use a chemical method to measure specific gravity however this is unreliable in veterinary patients and not recommended. Knowing the concentration of a urine sample helps to interpret results from the rest of the urinalysis. Sediment abnormalities may not be found in hyposthenuric (<1.007) or isosthenuric (1.007–1.015) urine due to a dilution effect.
Dipstick
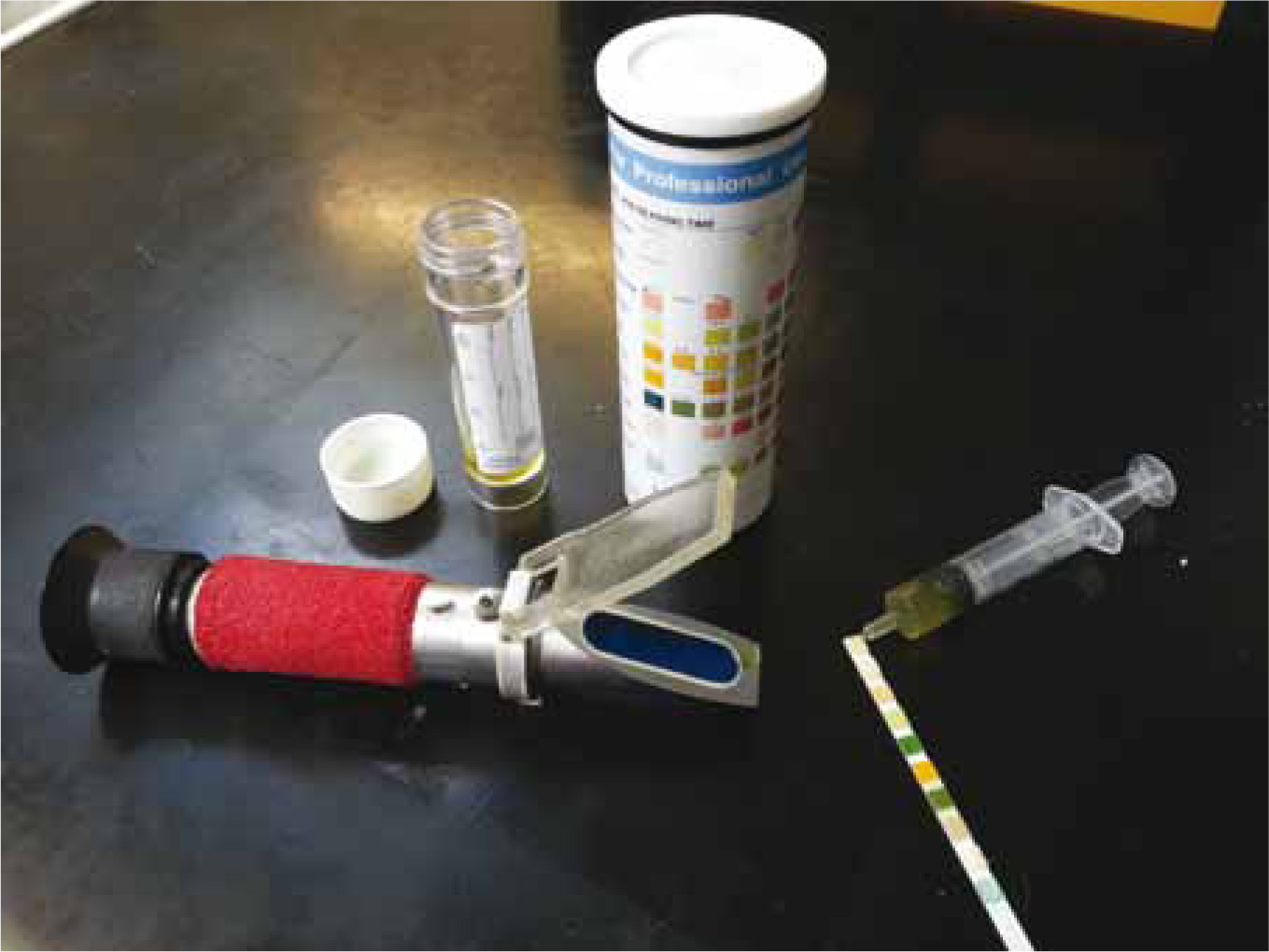
Quick and inexpensive, dipsticks are a useful diagnostic tool however they cannot provide a definitive diagnosis of infection (Figure 1). A pH >7.5 may indicate the presence of urease producing bacteria. Protein may indicate the presence of infection but is non specific. The presence of glucose and blood should also be noted. Nitrite and leukocyte esterase are not reliable for veterinary patients (Archer, 2010).
Sediment
Sediment examination is the most important step of the routine urinalysis. A specialised urine stain, such as Sedistain, added to sediment will facilitate cell identification. Bacteriuria, haematuria, pyuria and increased numbers of transitional epithelial cells in the urine sediment are indications of UTI. Bacteria visible in a cystocentesis urine sample indicates significant infection, bacteria in a free catch sample may be caused by contaminants which have proliferated. If there is suspicion of bacterial infection a culture and sensitivity will confirm diagnosis.
Culture and sensitivity
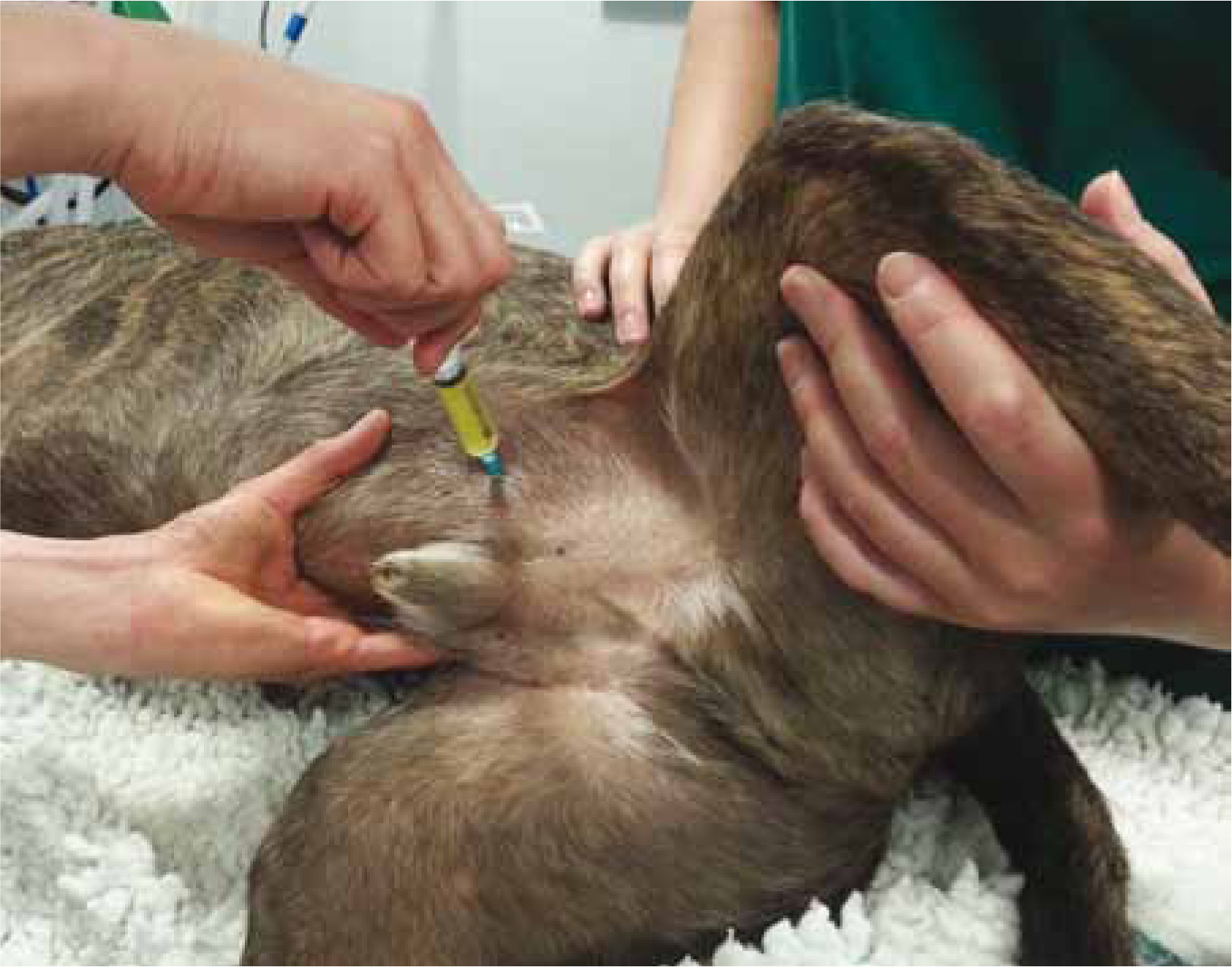
Five millilitres of urine collected by cystocentesis in a sterile plain tube should be sent to a laboratory as soon as possible for bacterial culture (Figure 2). The urine sample should be kept refrigerated and reach the laboratory within 24 hours of collection.
Quantitative analysis expressed as colony forming units (CFUs) per millilitre of urine must be interpreted knowing the method of urine collection. It is therefore important to always state the method of collection on a laboratory form. Urine bacterial counts >103 CFU are significant in a cystocentesis sample, catheterised samples will contain more contamination and the threshold of infection rises to >105 CFU. Bacterial contamination occurs in up to 85% of free catch midstream samples (Jagger, 2010). If no alternative method of collection is available, bacterial counts >106 CFU are associated with infection. Positive free catch samples should be interpreted with care; females are likely to have more contamination than males (Jagger, 2010).
Treatment and management
Empirical antibiotic selection
Uncomplicated UTIs are infections that are not associated with any detectable underlying disease. It is not always necessary to culture urine from these patients as a good response can be expected from an appropriate empirical antibiotic selection. An empirical treatment is one chosen from past experience of its beneficial effects. PROTECT, an initiative of the Small Animal Medicine Society (SAMSoc) to promote responsible antibacterial prescribing recommend practices use amoxicillin/clavulanate OR trimethoprim/sulfadiazine as their first line treatment for bacterial lower UTI (PROTECT poster, 2012).
Sensitivity testing
Complicated UTIs are infections that are associated with underlying deficits in the host defence mechanisms. These underlying deficits must be corrected to achieve infection resolution. Sensitivity testing is recommended for all animals that have received antibiotics within the preceding 6 weeks, those that have recurrent UTIs or are at risk of complications. Empirical antimicrobial therapy is indicated in most cases while awaiting culture and susceptibility results to relieve patient discomfort (Weese et al, 2011)
How long is long enough for antibiotic therapy?
Evidence-based recommendations for duration of antibiotic therapy are lacking in the veterinary literature. Patients with uncomplicated lower UTIs are typically treated with a 7–14 day course of antibiotics (Smee, 2013). A systematic review of antibacterial treatment of uncomplicated UTI in women found 3 days of antibiotic therapy to be similar to 5–10 days in achieving symptomatic cure. Longer treatment is more effective in obtaining bacteriological cure. Milo et al (2005) concluded treatment for 5–10 days could be considered for treatment of women in whom eradication of bacteriuria is important. Similar evidence is required in veterinary medicine if antibiotic usage is to be reduced without affecting patients' welfare.
Antimicrobial treatment should continue for 4–6 weeks in chronic infections, upper UTIs and where there is prostate involvement (Senior, 2007). Complicated and chronic UTI cases should have urine culture repeated 7 days after antibiotic therapy has started and 7 days after therapy has finished. Only a sterile sample is an indication of successful treatment. The prognosis for an animal with a complicated UTI as opposed to an uncomplicated UTI is always guarded if predisposing factors cannot be identified and eliminated.
Recurrent and resistant infections
Recurrent UTIs can be defined as either a relapse, indicating a failure of treatment or a reinfection, indicating an underlying deficit in the host defence mechanism (Smee, 2013).
A relapse of infection usually occurs within several days of cessation of treatment and involves the same bacterial species. Relapses may be due to inappropriate antibiotic selection, dose or duration, antibiotic resistance, lack of owner compliance or a nidus of infection (e.g. prostatitis, pyelonephritis, urolithiasis). Reinfection is a subsequent independent infection with another bacterium after the previous infection was cleared. Reinfection often indicates failure to eliminate the predisposing condition altering normal host defence mechanisms (Nelson and Couto, 2003).
Nursing care
Nurses play an important role in the prevention and treatment of canine UTIs. An awareness of predisposing conditions means nurses can educate clients on signs to monitor for and the potential consequences of UTI. Proactive urinalysis and culture in susceptible patients will aid diagnosis in early and occult disease. Familiarity with the urine sampling techniques discussed in this article and routine urinalysis is essential for good patient care.
Nursing care of paralysed and paretic patients should include maintaining good hygiene of the perineum to reduce urine and faecal scalding and reduce the load of potential uropathogenic bacteria. Urinary catheters should be placed with care to minimise bladder trauma and using an aseptic technique. Repeated catheterisation and the duration of indwelling urinary catheters should be minimised where possible. Indwelling catheters should be connected to a closed system, securely placed and urine drained every 2–4 hours. Closed systems sealed by a bung or connected to a sterile urine collection bag reduce urine scalding and the risk of infection (Bubenik and Hosgood, 2008).
For patients being managed at home it is important that owners are given a follow-up plan. When empirical antibiotic treatment is used owners should know what to expect and when to return to their veterinary practice for follow-up urinalysis. Owners should be told the importance of completing the entire antibiotic course, even if signs resolve early. The possibility of UTI recurrence should be communicated so that owners continue to observe their pets closely for symptoms.
Prophylaxis
Long-term, low dose, daily antibiotic treatment may be used to reduce the recurrence rate of UTIs in susceptible patients after resolution of an acute infection. There are currently no studies to support the use of prophylactic antibiotics for the prevention of canine UTIs. A Cochrane report looking at the efficacy and harms of long-term antibiotics to prevent recurrent UTI in children concluded that the benefit is small and must be considered together with the increased risk of microbial resistance (Williams and Craig, 2011).
Type-A proanthocyanidins found in cranberries inhibit E. coli attachment to uroepithelial cells, vaginal epithelial cells (Gupta et al, 2007) and to PVC and PTFE (Teflon), plastics commonly used in urinary catheters (Eydelnant and Tufenkji, 2008). Dogs receiving oral cranberry extract produce urine with anti-adhesion effects on E. coli (Howell et al, 2010). Smee et al (2011) found E. coli added to the urine of dogs receiving cranberry extract had a decreased ability to adhere to Madin-Darby Canine Kidney cells. Supplementation of proanthocyanidins does not select for antimicrobial resistance.
Live beneficial bacteria, probiotics, taken orally or applied topically to the urethra or bladder may be a future viable alternative to repeated doses of antimicrobial agents (Thompson et al, 2011). Probiotics and nutraceuticals may have an important role in treating and preventing UTIs. More research is required to verify the most effective nutraceutical protocols in small animal veterinary therapeutics.
Conclusion
The majority of canine UTIs are uncomplicated and resolve with appropriate antibiotic treatment. Patients with recurrent UTIs should be investigated for underlying conditions which may be affecting their normal defences against infection, e.g. chronic renal failure. If underlying conditions are not addressed complete resolution of infection is unlikely and may lead to complications such as pyelonephritis. Veterinary nurses should be aware of which patients are at risk of UTIs and be proactive in performing urinalysis and culture as many patients will not show symptoms of infection.
Antibiotics are the main form of treatment for canine UTIs; however the benefits of long-term low-dose antibiotic treatment for the reduction of recurrence rates must be weighed against the risk of microbial resistance.

