Registered veterinary nurses (RVNs) should have a basic understanding of the general small rodent anatomical and behavioural characteristics in order to identify compromised patients and contribute to emergency and critical care nursing plans (Table 1). For the purposes of this article the term ‘small rodent’ will refer to rodents below 500 g bodyweight (BW) and encompass the most commonly kept species, e.g. rat (Rattus norvegicus), mouse (Mus musculus), degu (Octodon spp.), gerbil (Meriones unguiculatus), and hamster (Syrian, Mesocricetus auratus; Siberian, Phodopus sungorus; Chinese, Cricetulus griseus).
| HAMSTER | RAT | MOUSE | GERBIL | DEGU | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All 4 species | Syrian | Russian (WW) | Russian (Campbell) | Roborovskii | Chinese | ||||||
| Lifespan (years) | Male (buck) | 1.5–2 | 1–3 | 2–2.5 | 3 | 2.5–3 | 3–4 | 2–3 | 3–4 | 7 | |
| Female (Doe) | 2–3 | 3–4 | 7 | ||||||||
| Adult body weight (g) | Male (Buck) | 85–130 | 19–45 (seasonal fluctuations-lowest in July) | 22–28 | 30–35 | 225–325 | 25–50 | 60 | 170–300 | ||
| Female (Doe) | 95–150 | 19–36 (seasonalas above) | 27–32 | 270–500 | 22–63 | 50 | 250 | ||||
| Dentition | I 1/1, C 0/0, PM 0/0, M 3/3 | - | - | - | I 1/1, C0/0, PM 0/0, M 3/3 | I 1/1, C 0/0, PM 0/0, M 3/3 | I 1/1, C 0/0, PM 0/0, M 3/3 | I 1/1, C 0/0, PM 1/1, M 3/3 | |||
| Respiratory rate (breaths/min) | 40–70 | 60–80 | 60–80 | 60–80 | 60–80 | 60–150 | 100–280 | 80–150 | ~40–100 | ||
| Heart rate (beats/min) | 250–400 | 300–460 | 300–460 | 300–460 | 300–460 | 250–450 | 500–600 | 250–400 | ~100 | ||
| Rectal temperature (°C) | 36–38 | 36–37.5 | - | - | - | 37.6–38.6 | 37–38 | 37.5–39 | 37.9 | ||
| Blood volume | 0.078 ml/g (3.9 ml/50 g) | - | - | - | - | - | 6 ml/100 g bw | 15 ml/100 g bw | 0.7ml/0g | 7 ml/100 g bw | |
| Daily water intake | 10ml/10g bw | - | - | - | - | - | 10 ml/100 g bw | 15 ml/100/g | 4–10 ml/100 g BW | 4–6 ml/100 g bw | |
| Food consumption | 15g/100 bw | - | - | - | - | - | 10 ml/100 g bw | 15 g/100 g bw | 8–9 g/100 g BW | 3–6 g/100 g bw | |
| Urine analysis | pH | 7.3-8.5 | - | - | - | - | 7.3–8.5 | 7.3–8.5 | 6.5–7.5 | Unknown | |
| Specific gravity | 1.034–1.058 | - | - | - | - | 1.022–1.070 | 1.034–1.058 | Highly concentrated | Highly concentrated | ||
| Consistency | Yellow (dilute milky yellow (thick) | - | - | - | - | Yellow to opaque/milky | Yellow (dilute) – milky yellow (thicker) | Clear yellowish | Yellow – Orange, thick | ||
| Sexual maturity (weeks) | Male (Buck) | 6–8 | 6–8 | 6–8 | 5–12 | 7–14 | 8 | 6 | 8–9 | 24 | |
| Female (Doe) | (Mating no earlier than 5 months) | 10 | 7 | 9–10 | 16–36 (4-9 months) | ||||||
| Length of gestation (days) | 15–18 | 18 | 18–20 | 22–30 | 21 | 20–22 | 19–21 | 24–26 | 87–93 (ave 90) | ||
| Litter size (pups) | 5–9 | 4 (up to 11!) | 4-8 | 4–6 (up to 10!) | 4–5 pups (up to 10!) | 6–16 pups | 8–12 pups | 4–6 pups | 1–12 (ave 5) pups | ||
| Normal birth weight (g) | 0.5–1.5 | - | - | 1.5 | - | - | 4–7 | 0.5–1.5 | 2.5–3.5 (BW) | 14 | |
| Activity | Nocturnal | - | - | - | - | - | Nocturnal | Nocturnal | Nocturnal | Diurnal | Diurnal |
| Hibernation | Yes | No | No but remain underground during winter | Partial hibernation — long periods of deep sleep | No | No | No | No | |||
Species characteristics
In general small rodents are relatively short lived, 2–4 years, although longer in the degu (7 years) and the effects of ageing may predispose individuals to critical conditions (O'Malley, 2005; Table 1). Their small body size can not only pose a challenge when handling and applying treatments, but also in ensuring their high metabolic demands are met, e.g. they are prone to hypoglycaemia following periods of anorexia. This high metabolism and small body mass to body surface ratio, also increases their sensitivity to environmental temperature fluctuations, e.g. hypothermia or hyperthermia. It is well documented in the literature that rodents exhibit typical ‘prey-like’ attributes and respond to olfactory cues from potential threats (e.g. dogs, cats, humans), which predisposes them to stresses faced in a veterinary hospital (Ferrero et al, 2011). This is an innate response necessary for survival (Bradley Bays, 2006). Predator avoidance strategies, coupled with the potential for a poor appreciation or late detection of an illness by the owner, often results in rodents presented to veterinary staff in a critically ill state.
Identifying patients requiring critical care intervention
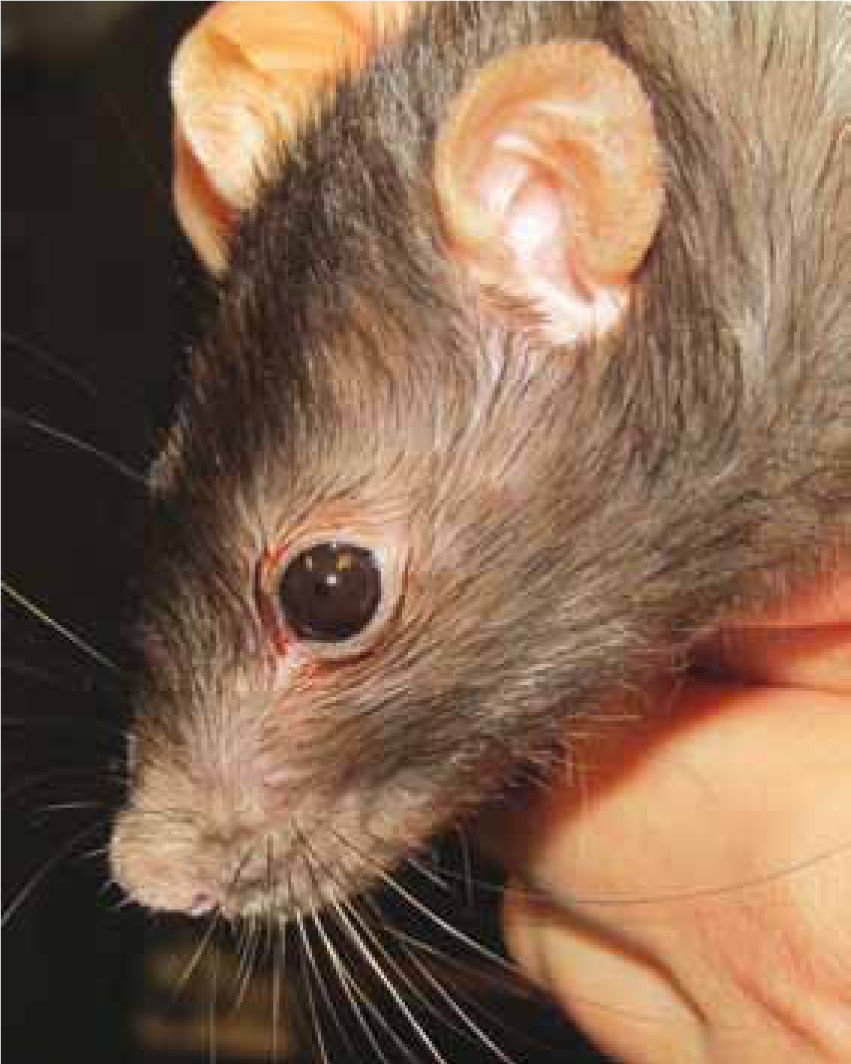
It is imperative to determine early on if the rodent is requiring critical or emergency assistance on initial examination and a full physical assessment may have to wait until the patient is more stable (Hawkins and Graham, 2007; Tamura, 2010). Debilitated small rodents may exhibit clinical signs such as hunched posture, piloerection of fur guard hairs (Figure 1), anorexia, general lethargy, chromodacryorrhea (Figure 2), laboured breathing, vocalisation with or without stimulation, shivering, ataxia and voiding abnormal faeces and urine (Miller, 2011). Immediate stabilisation should be provided with urgency by observing for any improvement in a quiet, darkened environment, and administering oxygen, heat therapy where indicated, as well as food and water.

For further discussion on the aetiology of the commonly encountered illnesses of small rodents please refer to the literature provided (Hawkins and Graham, 2007; Kling, 2011; Lichtenberger and Lennox, 2012).
Common clinical techniques
Venepuncture
When acquiring a blood sample in healthy rodents it is advised to take no more than 10% of the blood volume (blood volume = 7% BW) or 1% of BW (Wesche, 2009; Box 1; Table 2). In sick individuals it is suggested to reduce the sample volume, e.g. 0.5% of BW (Hawkins and Graham, 2007). In general it is advisable to take blood under general anaesthetic in such small patients, although rats may tolerate conscious bloods (author's experience). Where a general anaesthetic is contraindicated and carries too large a risk to survival in debilitated patients, then blood sampling should be postponed until the patient is more stable.
| Sample | Measurement | Normal parameters | ||||
|---|---|---|---|---|---|---|
| Mice | Rats | Hamster | Gerbil | Degu | ||
| Packed Cell Volume (PCV) | 35–40% | 37.6–50.6% | 45–50% | 35–45% | 26–54% | |
| Total protein (TP or serum solids) | 35–72 g/l | 56–76 g/l | 52–70 g/l | 43–125 g/l | 56–61 g/l | |
| Blood glucose | 3.3–12.7 mmol/l | 4.7–7.3 mmol/l | 52–70 g/l | 2.8–7.5 mmol/l | 4.1–4.6 mmol/l | |
| Blood Urea Nitrogen (BUN) | 6.1–10.0 mmol/l | 2.4–3.4 mmol/l | 4.28–9.28 mmol/l | 6.1–9.6 mmol/l | 4.1–8.3 mmol/l | |
| Alanine aminotransferase (ALT) | 26–77 IU/L | 17.5–30.2 IU/l | 22–128 IU/l | - | 3-12 IU/l | |
| Creatinine | 27–88 µmol/l | 17.6–70.1 µmol/l | 35.4–88.4 µmol/l | 53–124 µmol/l | 18–44 µmol/l | |
| Calcium | 0.8–2.0 mmol/l | 1.3–3.2 mmol/l | 5.3–12 mmol/l | 0.93–1.55 mmol/l | 2.55 mmol/l | |
| Phosphorus | 1.94–3.56 mmol/l | 1–3.6 mmol/l | 0.96–3.19 mmol/l | 0.93–1.55 mmol/l | 2.00 mmol/l | |
| Urine analysis | Urine specific gravity (using a refractometer) | 1.034–1.058 g/l | 1.022–1.070 g/l | 1.014–1.060 g/l | >1.130 g/l | 1.123 g/l |
| pH | 7.3–8.5 | 7.3–8.5 | 5.1–8.4 | 6.5–7.5 | - | |
| Other notable chemical analysis | Proteinuria and small amounts of ketones | Proteinuria in old rats | Proteinuria and small amounts of ketones | - | - | |
| Ectoparasites | Mites May be a normal occurrence in low numbers | Myobia musculi, Myocoptes musculinus, Radfordia affinis | Radfordia ensifera | Demodex spp.(aurati) | Demodex meroni (rare) | Demodex |
| Lice | Polyplax serrata (rare) | Polyplax spinulosa | ||||
The most commonly used sites for blood sample are cranial vena cava, lateral saphenous and jugular veins (Jenkins, 2008; Tamura, 2010). Rats have prominent, easily visible lateral, dorsal and ventral tail veins which can be accessed in calm rats (Bober, 1988), with local anaesthesia, e.g. lidocaine or Emla cream®, and heat applied to the base of the tail (Hawkins and Graham, 2007) to facilitate sampling. This technique should not be attempted in degus or gerbils because of the risk of tail de-gloving injuries.
Urinalysis
The urine collection techniques are limited in small rodents because of their small size, but free catch samples can be acquired from non-absorbable surfaces such as briefly placing the patient in a perspex carrier (author's experience). The sample will most likely be contaminated, but still useful for some diagnostics (e.g. specific gravity, and chemical analysis) (Jenkins, 2008; Tamura, 2010; Table 2).
Faecal examination
A general evaluation of the appearance and amount of faeces passed from a patient can be done. Wet preparation smears and floatation, Gram staining or Diff-Quik® can also be performed in small rodents to highlight the presence of bacteria, parasites or blood.
Hospitalisation and critical care nursing
Hospitalisation of critically ill small rodents requires some planning and awareness of the species being dealt with to optimise treatment success and patient welfare.
The ward environment should provide minimal stress from external cues from potential predators. Therefore, it should be maintained quiet, possibly darkened, with temperatures between 26–28°C (Hawkins and Graham, 2007). Placing a small towel in front of the enclosure could help minimise disturbance. Staff should acknowledge that most small rodents are nocturnal species, and resting during daytime hours is essential.
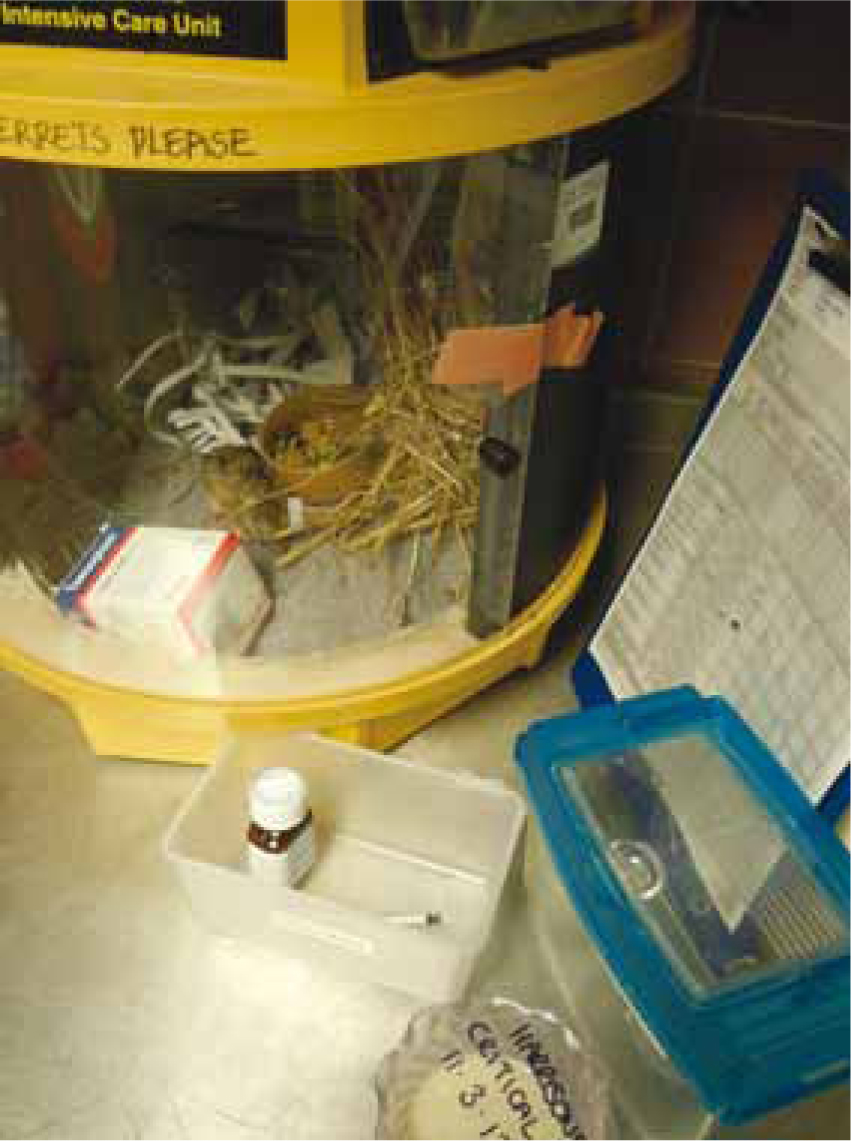
The hospital cage should be secure and safe with appropriately small mesh, to prevent escapees and allow choices for the patient to access small concealed areas, e.g. ‘hide/nest boxes' with plenty of moveable bedding material (Figure 3; Litchenberger and Hawkins, 2009). To maintain familiarity and potentially minimise stress, the patient's own bedding, and other furniture can be used. Fresh water can be offered in bottles and ceramic bowls to prevent spillage and allow for any preference (Hagen et al, 2014).

Infection control
As with other species and clinical environments, it is important to maintain excellent infection control and hygienic standards to minimise risks of zoonotic disease transmission. The occurrence of rodent zoonoses is low, but the most notable are lymphocytic choriomeningitis virus (LCM) and hantavirus (Tamura, 2010; Hollamby, 2009). As most are transmitted via urine and saliva, standard hygienic practices such as washing hands after handling, wearing examination gloves, discarding used bedding and disinfecting contacted surfaces, should be adequate to prevent transmission of most pathogens.
Fluid therapy
Small rodents require maintenance fluids at 80–100 ml/kg BW/day (Tamura, 2010; Table 1). Where dehydration is suspected or anorexia is present, patients should be supported with fluid therapy and any losses replaced. As with other species, for every 1% dehydration, 10 mls/kg bodyweight is required to be replaced and the following calculation is advised:
% dehydration x BW (kg) x 1000 ml/litre = fluid deficit (in millimeters)
(Lichtenberger, 2012; Box 2). Patients are considered 5% dehydrated when clinical signs such as weakness, increased thirst and tacky mucous membranes are seen (Hawkins and Graham, 2007). Suitable crystalloids include compound sodium lactate or lactated ringers solution.
Oral (per os (PO))
There are a variety of convalescent formulas that are palatable to many rodent species, can be administered orally and considered a useful alternative technique for fluid administration (Figure 4).

Intravenous (IV)
Catheter placement is very challenging with small rodent species. It is not usually successful in most rodent species due to their small size, but it is achievable in rats and degus using the cephalic or lateral saphenous veins. Local or general anaesthesia may be required (author's experience).
Intraosseous (IO)
IO catheterisation can prove useful in collapsed patients to provide a secure and efficient route for fluid and drug administration. The standard landmarks and aseptic procedure used in other species enable successful and hygienic placement. The proximal tibia (at the tibial crest), proximal humerus and proximal femur (greater trochanter) are common insertion sites (Figure 5). Smaller rodents such as gerbils, hamsters and mice can be catheterised using hypodermic needles although a sterile wire should be used to remove any clot or bone occlusion (Lennox, 2012).
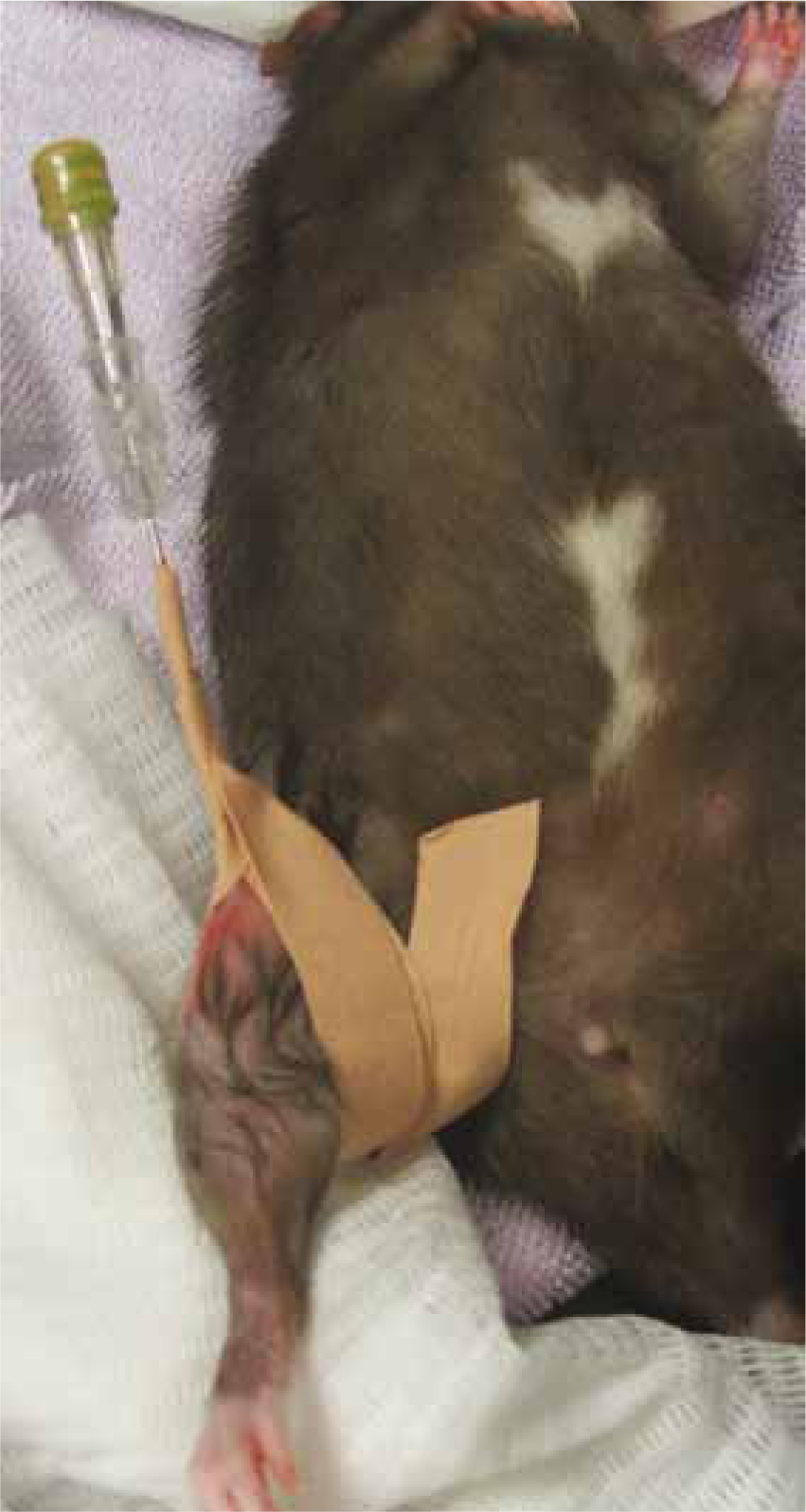
Subcutaneous (SC)
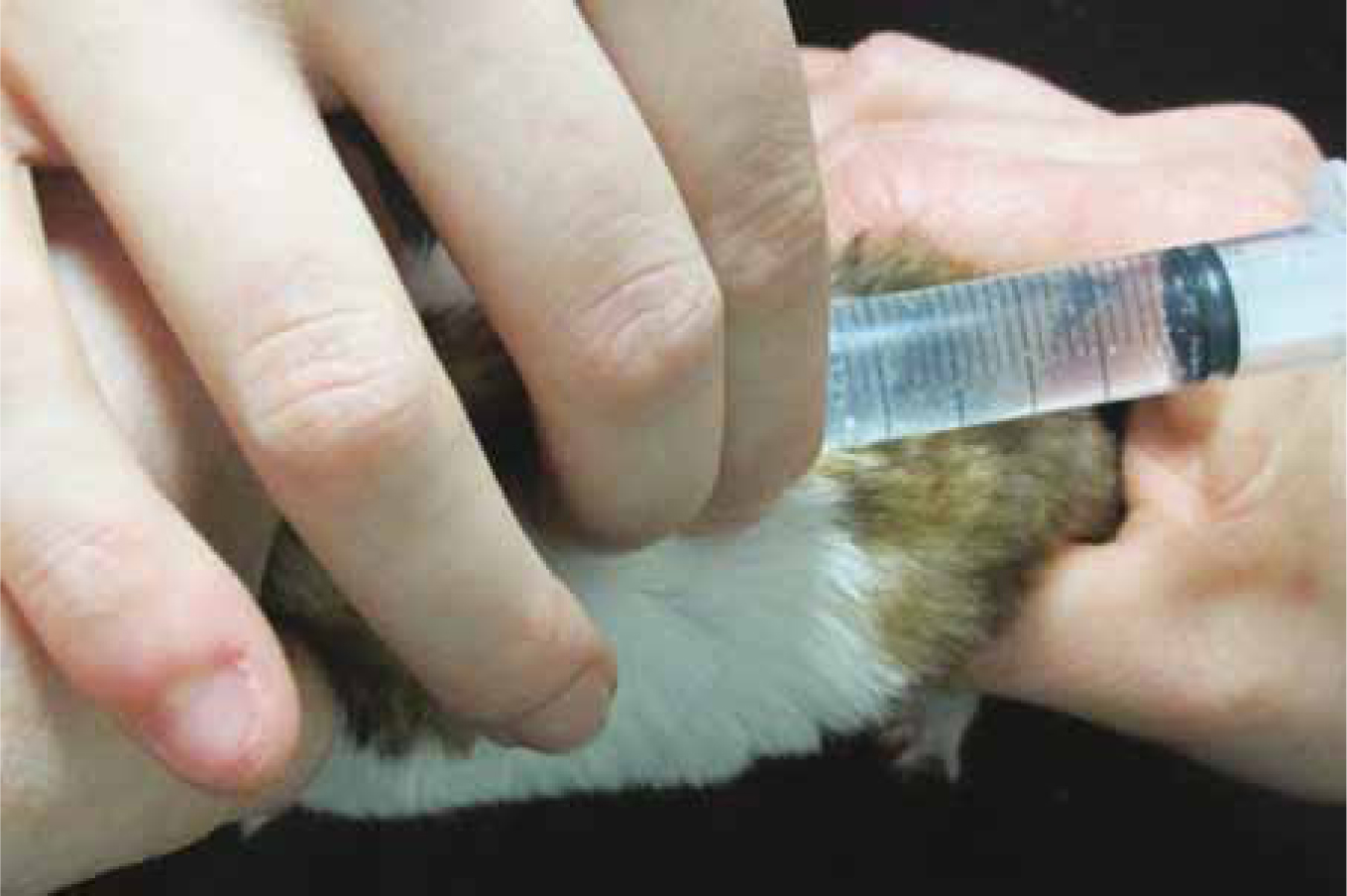
This route is the least invasive, most convenient and easily accessible for rapid fluid administration with minimal handling. Boluses should be warmed to body temperature (38–39°C) (Lichtenberger and Lennox, 2012). Relatively large boluses may be combined with hyaluronidase (Figure 6; Hyalase ® 1:100 dilution), which aids the dissipation of fluids through tissue.
Nutritional therapy
It is vital to appreciate the species-specific dietary requirements as well as individual preference. There are a variety of species-appropriate convalescent diets (or critical care formulae) commercially available that appear highly palatable to many rodents allowing for just gentle encouragement to eat off a spoon, bowl or droplets off the end of a syringe (Figure 4; author's experience). Omnivorous formulae should be fed to rats, whereas herbivorous diets are suitable for degus. Where force feeding is indicated (e.g. repeated refusal of food items offered), small rodents can be a challenge to restrain and sensitivity is required regarding the perceived level of stress this can create (Hawkins and Graham, 2007).
Thermo/heat therapy
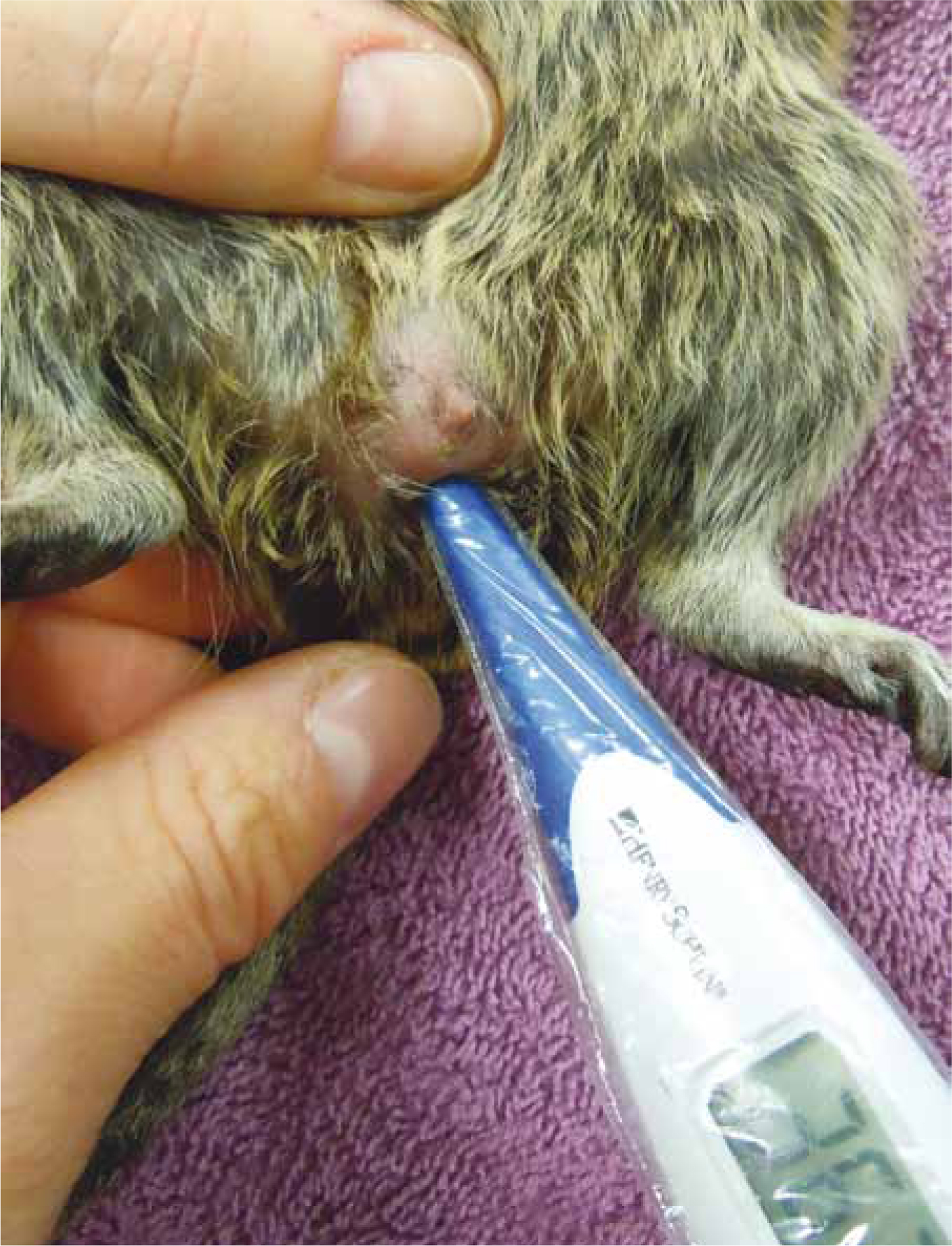
Body temperature assessment and regulation will be a crucial part of managing critically ill rodent patients, as they are prone to hypothermia or may suffer from heat stress in heated environments (Lichtenberger and Lennox, 2012). It can be challenging to acquire rectal temperatures from rodents weighing <100 g bodyweight, and assessments may need to be based on patient behaviour and body surface temperature using touch or infrared non-touch laser thermometers (Figure 7). However, some companies have developed fine and flexible rectal probes that can be tolerated. Incubators, or brooders, can provide a controllable environmental temperature for critically ill rodents, as well as facilitate oxygen therapy and nebulisation (Figure 8; Evans, 2006; Lichtenberger, 2012). Other useful heating aides come in the form of warmed fluids, microwavable head pads, cherrystone bags or even examination gloves filled with warm-water, although recumbent patients, that are not able to move, should be carefully monitored and not placed directly on top of heat sources.
Medication: drugs and route of administration
Many critically ill rodent species will voluntarily take PO medications that are sugary, or food flavoured (Figure 4; author's experience), but other useful medication routes are SC, or IO routes (Tamura, 2010; Table 3). Intramuscular route for drug administration is generally not advised as it can be difficult because of the relatively small body size and cause irritation to tissues, which could lead to self-traumatisation. Intraperitoneal (IP) can be a useful, quick route, but care should be taken to avoid damage to internal organs especially in a small, wriggly patient.
| Drug type | Drug name | Dose | Administration route | Frequency | Example treatment indicated |
|---|---|---|---|---|---|
| Analgesia (should be pre-emptive where possible) | Buprenorphine | 0.04 mg/kg | SC, IV | q 8–12 hours | Trauma, has some sedative effects |
| Butorphanol | 1–5 mg/kg (G, H), 0.2–2 mg/kg (R),1–5 mg/kg (M) | SC | q 4 hours (G, H, M), q 2–4 hours (R), | Can cause profound sedation and used in pre-GA regimen | |
| Meloxicam | >0.5 mg/kg (G, H), 1–2 mg/kg (R), 1–5 mg/kg (M) | PO, SC | q 24 hours | NSAID | |
| Morphine | 2–5 mg/kg (G, H, R), 2–10 mg/kg (M) | SC | q 4 hours | Short duration of analgesia, used peri-operatively | |
| Carprofen | 5 mg/kg (G, H), 2–5 mg/kg (R, M) | SC (G, H), PO, SC, (R, M) | q 24 hours | NSAID | |
| Tramadol | 5–10 mg/kg (G, H), 5–20 mg/kg (R), 5–40 mg/kg (M) | PO (G, H), PO, SC, IP (R), SC & IP (M) | q 12–24 hours | ||
| Anaesthetics (references provide more options) | Atipamezole | 0.1–1.0 mg/kg. 1:1 volume of medatomindine or dexmedatomidine | IV, IP | Reverses Medatomidine | |
| Buprenorphine | 0.04 mg/kg | SC, IV | q 8–12 hours | Trauma, has some sedative effects | |
| Butorphanol | 0.2–0.4 mg/kg | SC | q 8 hours | Can cause profound sedation and used in pre-GA regimen | |
| Dexmedetomidine | 0.015–0.5 mg/kg | SC, IP | Sedation | ||
| Fentanyl/Fluanisone | 0.5–1.0 ml/kg | SC | One off treatment | Sedation and immobility good for radiographs or non-invasive procedures | |
| Medatomidine and Ketamine | 0.5–1.0 and 50–75 m/kg | SC | One off treatment | Can cause muscular contraction and pupil dilation. | |
| Midazolam | 1.0–2.5 mg/kg | IV, SC, IP | Sedation | ||
| Lidocaine 2% & Bupivicaine 0.5% | L: 1–2 mg/kg |
Periosteum, S/C, splash on wounds or prior to suturing. | Periosteum, S/C, splash on wounds or prior to suturing. | IO catheter placement, reduce risk of self-trauma when recovering from surgery | |
| Inhalant gases (Isoflurane or Sevoflurane) | Used to effect stating at 3%, reducing to 1–2% as maintenance | Induction chamber followed by mask prior to intubation | N/A | In stressed individuals for diagnostic procedures | |
| Antibiotics | Cefalexin | 25 mk/kg (G, H) 60 mg/kg (M) 15 mg/kg (R) | SC(G, H) PO(M), | q 24 hours (G, H, M), q 12–24 hour (R) | |
| Enrofloxacin | 5–20 mg/kg | PO, SC, | q 12 hour | ||
| Metronidazole | 20 mg/kg (G, H) 10–40 mg/kg (M, R) | PO | q 12 hours (H, G) |
||
| Gut stimulants | Domperidone | ||||
| Cisapride | 0.1–0.5 mg/kg | PO | q 12 hour | Bloat, antiemetic | |
| Metaclopramide | 0.2–1.0 mg/kg | PO, SC, | q 12hour | ||
| Gut protectants | Ranitidine | 3.5 mg/kg | PO | q 8–12 hours | Gastric ulcers |
| Sucralfate (best given on an empty stomach) | 25–100 mg/kg | PO | q 8–12 hours | Oral, gastric, duodenal ulcers | |
| Omeprazole | 4 mg/kg | PO | q 24 hours | Gastric and duodenal ulcers | |
| Ectoparasites | Fipronil (not licensed for small rodents) | 7.5 mg/kg | Topical between shoulder blades | q 30–60 days | Fleas and ticks only |
| Ivermectin | 0.2–0.4 mg/kg | SC | q7–14 days | Ectoparasites | |
| Emergency | Adrenaline | 0.01 mg/kg | SC, IV, ITracheal | One off treatments | Cardiac resuscitation |
| Atropine | 0.02 mg/kg | SC, | Corrects bradycardia, reduces mucous production, organophosphate toxicity | ||
| Diazepam | 1.0–5.0 mg/kg | IV, Rectal | Anticonvulsant, muscle relaxant | ||
| Doxopram | 2 mg/kg | IV, SC, IP, Sublingual | Stimulate respiration | ||
| Glycopyrrolate | 0.02 mg/kg | IV, SC | Corrects bradycardia, longer duration than atropine | ||
| Lidocaine | 1–2 mg/kg (no more than 4 mg/kg) | SC, Topical | Incision sites to improve anaesthesia and recovery, blood samples and IO catheter placement | ||
| Naloxone | 0.02 mg/kg | IV | Narcotic (opiod) antagonist. Increases blood pressure |
G = gerbil, H = hamster, R = rat, M = mouse, SC = subcutaneous, IV = intravenous, PO = per os, IP = intraperitoneal
Pain recognition and analgesia
Identifying the presence and severity of pain sensation in small rodent patients can be particularly challenging. Daily behavioural changes (e.g. vocalisations, anorexia, scratching or biting a focal point of irritation or hiding), including eating habits, are often the first indicative signs of pain that can be observed by the owner and support the need for thorough history taking during consultation (Evans, 2006; Hrapkiewicz and Medina, 2007; Mayer, 2007; Joslin, 2009). Small rodents are fastidious groomers, consequently, the appearance of an unkempt coat, piloerection of guard hairs and chromodacryorrhea could indicate discomfort (Figures 1 and 2). There are a variety of analgesic agents suggested in the literature for use in small rodents which may be used in most of the critical conditions seen (Table 3).
Oxygen therapy
In small rodents demonstrating dyspnoea, weakness, cyanotic mucous membranes or ‘open-mouth breathing’, 100% oxygen provision may be indicated. Oxygen can be provided by placing the rodent in an oxygen tent, incubator or temporarily placing the cage in a plastic bag. Oxygen humidifiers attached to a piped oxygen outlet can be used to deliver oxygen. It is possible to provide immediate 100% O2 via a face mask, breathing circuit and anaesthetic machine until the patient's condition has improved. Pulse oximeters can be useful in dyspnoeic patients, and placed on the paws or tail base, although some species can be a challenge to acquire a reading due to small body size or dark skin. Pulse oximeter units should be set and able to read up to 350 beats per minute.
Cardiac and respiratory arrest
When respiratory and/or cardiac arrest occurs, the response from the team should be immediate and efficient and follow the same principles as used in dog and cat medicine. Emergency drugs should be precalculated or drawn up for high risk patients (Table 3). In degus and rats intubation can be achieved by using a cat urinary catheter (4f) or IV catheters (14 + 18G) and either laryngoscope or otoscope that the tube can pass over (Hawkins and Graham, 2007; Richardson and Flecknell, 2009). At the very least the airway should be determined to be clear of any food debris or secretions, the heart listened to, and oxygen provided. When respiratory arrest is evident, gently raise and lower the abdomen, to move the weight of the abdominal contents against the diaphragm and emulate a breath to encourage spontaneous breathing (author's experience). Chest compressions in response to cardiac arrest should be performed very carefully between thumb and forefinger, so as not to damage the rib cage or lungs.
Conclusion
The assessment and care of pet small rodents requires patience, species-specific consideration, and keen vigilance by the veterinary team. RVNs should be sensitive to the requirements of small rodents in captivity and appreciate the urgency of support required when these patients are presented showing clinical signs of illness and in a compromised state. The planning and effectiveness of critical care in these species will greatly be governed by a methodical approach to initiatives used in more commonly encountered species and how they can be adapted to reach the same goals of preserving life and relieving suffering.

