Recent expansion of the lungworm Angiostrongylus vasorum from well known endemic areas of the UK and elsewhere (Morgan et al, 2009), along with the severity of clinical signs associated with infection in some dogs, has caused concern among the practising veterinary profession (Helm et al, 2010) and increased interest in parasitological diagnosis. However, it is easy to forget that besides Angiostrongylus vasorum, a number of other, less common lungworms can infect dogs namely Crenosoma vulpis, Filaroides osleri, F. hirthi and Eucoleus aerophilus. The life cycles of these parasites, their pathogenicity, and the risk factors for infection and reinfection are complex and varied and in many cases unclear, but knowing which species are involved in a clinical case can be very valuable, not least because specific veterinary advice and treatment is required.
Diagnosis may, in general, be indicated by radiography, bronchoscopy, clinical signs and haematology, and, in the case of A. vasorum, from an understanding and experience of local prevalence. But in order to make a definitive diagnosis, the parasite must be recovered and identified to species level. Until recently, this relied exclusively on microscopic examination of larvae and eggs. The advent of highly specific laboratory tests using the polymerase chain reaction (PCR) and antigen capture enzyme-linked immunosorbent assay (ELISA) has increased options for laboratory diagnosis of A. vasorum (Verzberger-Epshtein et al, 2008; Jefferies et al, 2009, 2011; Schnyder et al, 2011). This includes diagnosis using blood samples. However, diagnosis by larval recovery and microscopic identification still has a place in veterinary practice: it can achieve similar levels of sensitivity and specificity to more recent complex tests, can yield results more quickly if conducted in house, and unlike highly specific laboratory tests will detect the full range of lungworms that might be implicated in disease. Parasites can be recovered from faeces and in some cases by broncho-alveolar lavage (BAL) and from sputum, and the equipment and training requirements are modest. This article aims to review methods for the recovery of eggs and larvae of lungworms of dogs from clinical samples, which are suitable for the practice laboratory. Issues relevant to diagnosis using these methods are illustrated through a series of example cases.
Methods for egg and larval recovery
Standard methods for parasite egg recovery from faeces generally involve a dilution step, followed by flotation in saturated solutions of various salts (including sodium chloride). Where many eggs are present, they may be detected by direct wet smear, whereby a small amount of faeces is mixed with physiological saline on a microscope slide and a cover slip placed on top. Flotation and smear techniques can sometimes detect first stage lungworm larvae (L1), but sensitivity is poor, and more usually these are recovered from faeces and concentrated using a modified Baermann's technique, before being transferred to a glass slide so that their morphological detail can be examined under the high power of a compound microscope. An illustrated guide to the identification of lungworm larvae in dogs has been published recently (McGarry and Morgan, 2009). Special care should be taken with faecal samples collected from the ground, which could be contaminated with free-living nematodes. Also dogs that have the opportunity to scavenge could contain eggs and larvae of nematodes parasitic in other species such as livestock, which can pass through the gut intact and be found in the faeces.
The Baermann apparatus consists of a plastic funnel with a clipped rubber tube at the base; meshed conical gauze fits inside the funnel which is lined with a milk filter. The test faecal sample (1–3 g) is placed in a tea strainer inside the funnel (Figure 1) and flooded with water. The sample is gently broken up and mobile Lis migrate out of the sample and gravitate to the end of the clipped tube; the first few drops taken are collected directly into a Petri dish and screened under a stereo microscope. Various modifications of this apparatus are in common use, including tapering conical beakers, with larvae recovered from the bottom using a pipette, and replacement of the tea strainer with a bag of gauze (Koch and Willesen, 2009). Using larger faecal samples might also increase sensitivity. However, in clinical cases, larvae, when present, are often abundant, so refinements in the interests of slight gains in sensitivity are probably irrelevant in practical terms.

For species identification, L1 in a small drop of suspension must be carefully picked up with a pipette and transferred to a glass slide and a cover slip placed on top. This method is most sensitive once live L1 have had time to leave the faecal mass. The best time to read the test is around 8–24 hours after setting it up, hence it is ideally left to stand overnight (Morgan and Shaw, 2010). This has obvious disadvantages, in terms of a delay in obtaining test results — the time between sampling, test examination and reporting can be several days, especially if the sample is referred on to an external laboratory. Lungworms can cause acute life threatening disease and a diagnosis may be urgent. Reporting delays can therefore be highly problematic. Samples may be processed much more quickly by examining a wet faecal smear rather than Baermannizing the sample, but test sensitivity is compromised: Humm and Adamantos (2010) showed a low sensitivity of 61% for A. vasorum, when compared with the Baermann method. The Baermann test can also be read after 1 hour, and detects around 80% of cases that are positive after 8–24 hours (Morgan et al, 2010). A practical approach for diagnosis of urgent cases in the practice laboratory might therefore be to conduct a direct smear while setting up a Baermann's test. In negative cases, an attempt to recover larvae can then be made after 1 hour, with the sample left in place if still negative and a further attempt made the next day. Unfortunately, it has been shown that for A. vasorum the sensitivity of a single Baermann's test is low (Koch and Willesen, 2009), so if clinical suspicion remains high despite a negative result, treatment might still be warranted.
When BAL material is available, the sample is usually examined directly in a Petri dish, and mucopurulent samples may be diluted with saline if necessary.
Case studies
The following case studies referred to the School of Veterinary Science, Liverpool University highlight the above physical problems, and issues with diagnostic interpretation when faecal or BAL samples are examined.
Case one: A. vasorum — use of salt flotation/concentration
Case one was a 2-year-old dog with tachycardia, vomiting and diarrhoea in November 2010. Routine screening for worm eggs and protozoal cysts had been requested by the referring practice, but during routine parasitology L1 were found incidentally, and these were subsequently identified as A. vasorum. The routine procedure for faecal analysis involves two stages: initially a direct wet smear is made for cysts of parasitic protozoa, followed by a semi-quantitative flotation/concentration technique for helminth eggs using saturated sodium chloride, specific gravity 1.12. For the latter, a pea-sized amount of faeces is placed inside a 7 ml plastic bijou bottle which is then half filled with saturated sodium chloride solution and emulsified. Further salt solution is added dropwise to the bottle so that a meniscus forms, at which point a cover slip is immediately placed on top. After five minutes, the cover slip is quickly but carefully removed and placed onto a glass microscope slide. In the case described here, L1 were not seen in the wet smear but were numerous following flotation/concentration and remained intact after 30 minutes in the solution (Figure 2). L1 were also numerous in sediment examined from a Baermann's test run overnight.
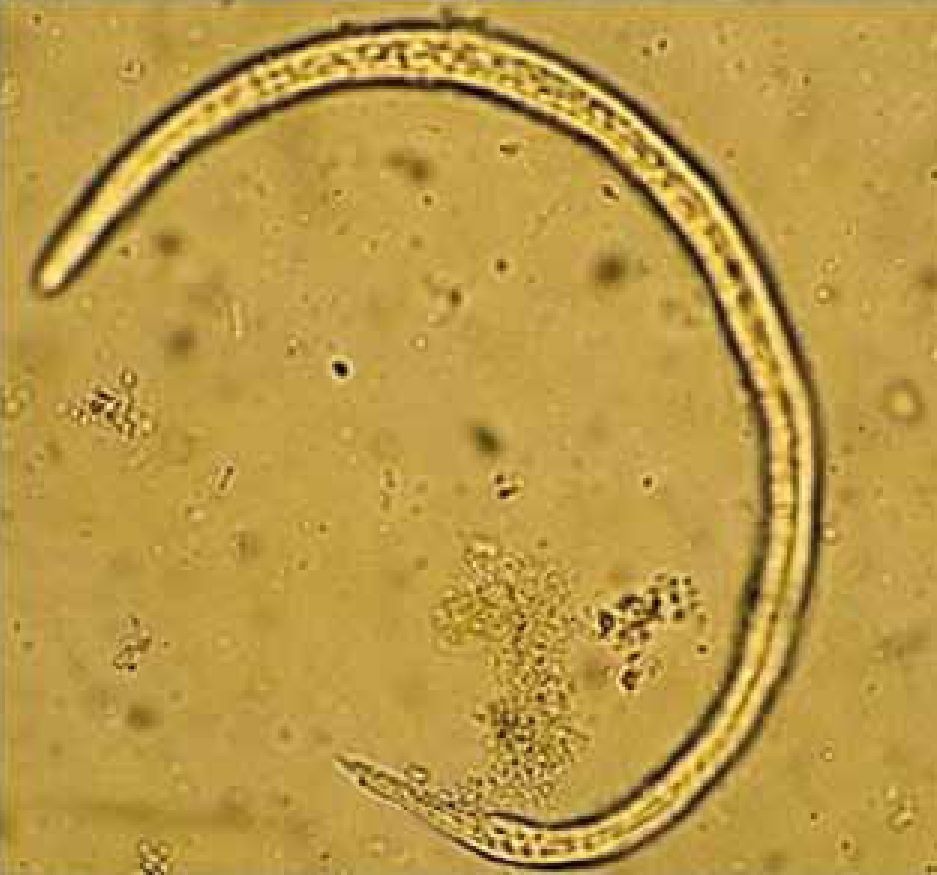
This dog had never travelled outside the urban regions of Birkenhead, Wirral and the case appears to be the first from the Wirral Peninsula, confirming the sporadic occurrence in pet dogs around or within large urban areas in northern England and Scotland (Helm et al, 2009; Yamakawa et al, 2009). This case raises the possibility of using salt flotation/concentration techniques as a one-step procedure to aid lungworm detection and identification.
Cases two and three: A. vasorum — intermittent shedding of L1
Case two was a 9-year-old dog from Essex with a cough and dyspnoea after exercise. Radiographs were striking with changes presenting as a mixture of a moderate to marked generalized broncho-interstitial pattern, with localized alveolar infiltrates predominantly in the caudal fields giving an overall appearance that resembled pulmonary metastases. The lesions are due to large numbers of L1 and eggs in the lungs, and associated inflammatory reaction. Examination of a wet faecal smear and a Baermanised sample revealed very few larvae of A. vasorum, and very surprisingly, no larvae at all could be found in the BAL. A second sample of BAL and faeces analyzed using the same approach but taken a day later revealed numerous L1.
Case three concerned two siblings in the same household. The first dog, a 1 year old, had no apparent respiratory illness but was suffering from bleeding and anaemia and subsequently died. A. vasorum adult worms were recovered as an incidental finding at post mortem. As an immediate consequence, two faecal samples from the second, asymptomatic dog were taken 2 days apart and Baermannised. The first sample appeared negative but scanty numbers of A. vasorum larvae were found in the second sample.
The timing of sampling is important. These cases confirm that L1 of A. vasorum intermittently appear in the faeces (and BAL) of infected dogs (Oliveira et al, 2006) and that sensitivity can be increased by analyzing sequential samples (Koch and Willesen, 2009). Case three highlights the importance of follow-up testing of dogs from the same household, which share the same immediate environment and exposure to infected snail and slug populations.
Cases four and five: other lungworms
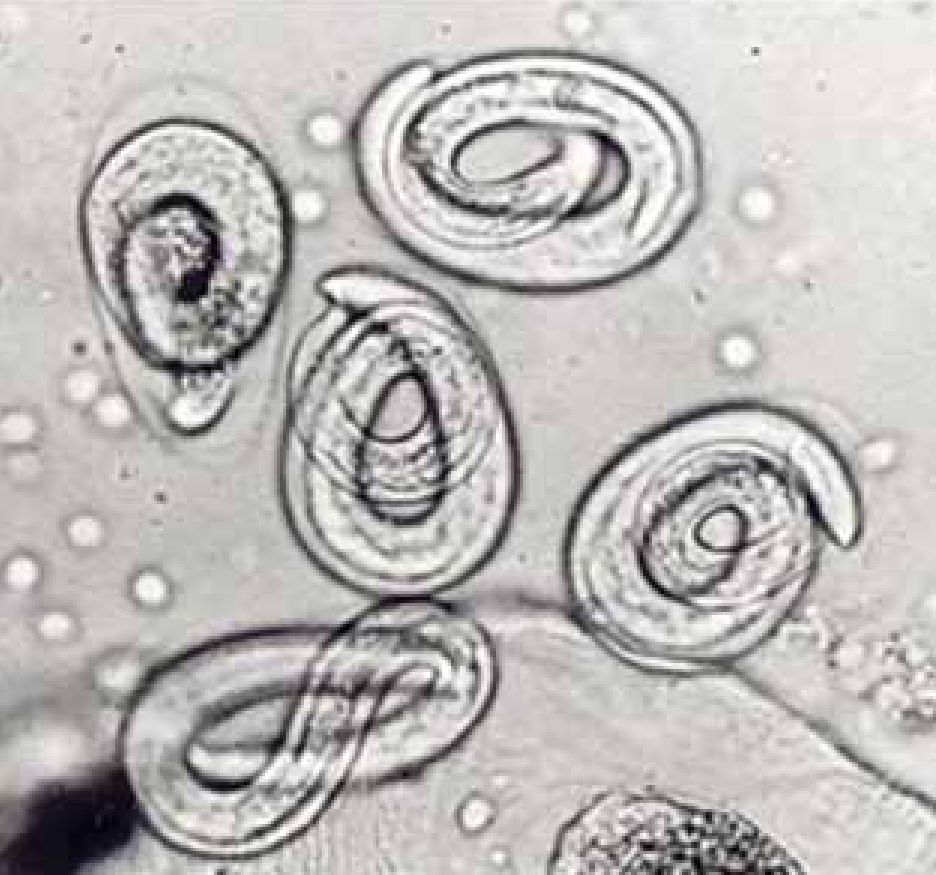
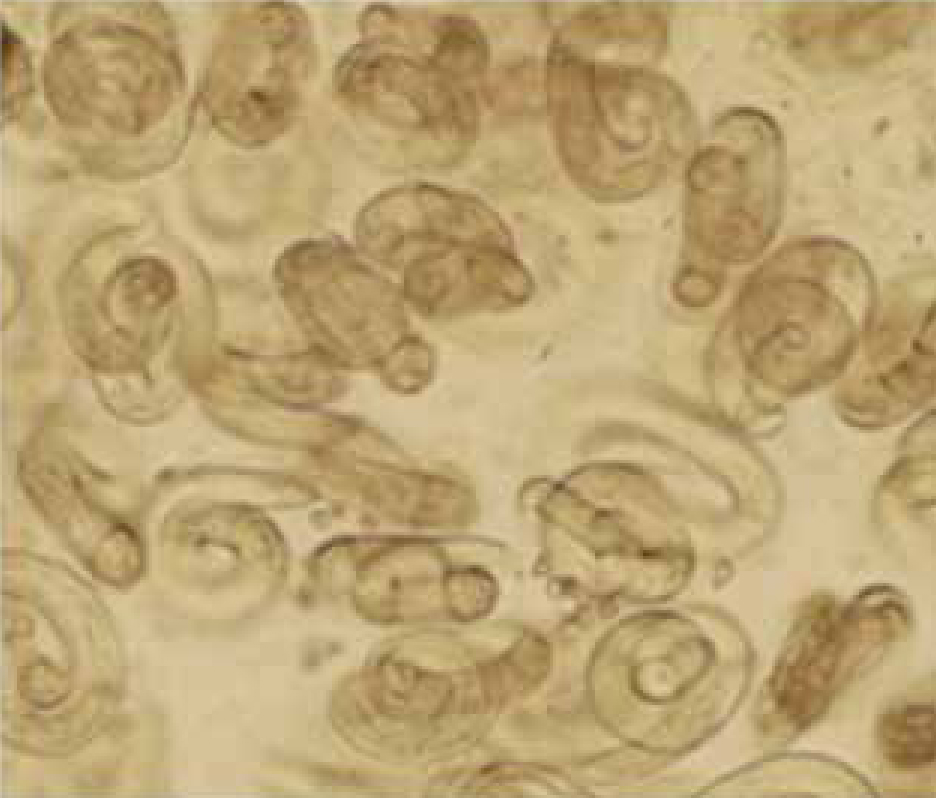
There are very few published case studies from the UK concerning Crenosoma vulpis or Filaroides osleri, perhaps due in part to difficulties in finding and identifying L1 and eggs, respectively. The limited number of clinical diagnoses for these species in the authors' laboratories suggests that for both these species, larvae/eggs are more likely to be seen in BAL rather than faeces. In one case of crenosomiasis (Reilly et al, 2000), for example, the adult worms which sit in mucus in the large airways were not seen by bronchoscopy, but L1 and eggs were very plentiful in BAL (Figure 3). Baermannised faecal samples from the same dog, however, proved negative. F. osleri has been diagnosed in the authors' laboratory on only two occasions and both were negative on Baermannisation. One of these cases was a 2 year old with a chronic cough showing numerous nodules in the main airways visible on bronchoscopy. Adult worms were dissected from biopsied tissue and there were numerous eggs present in BAL only (Figure 4).
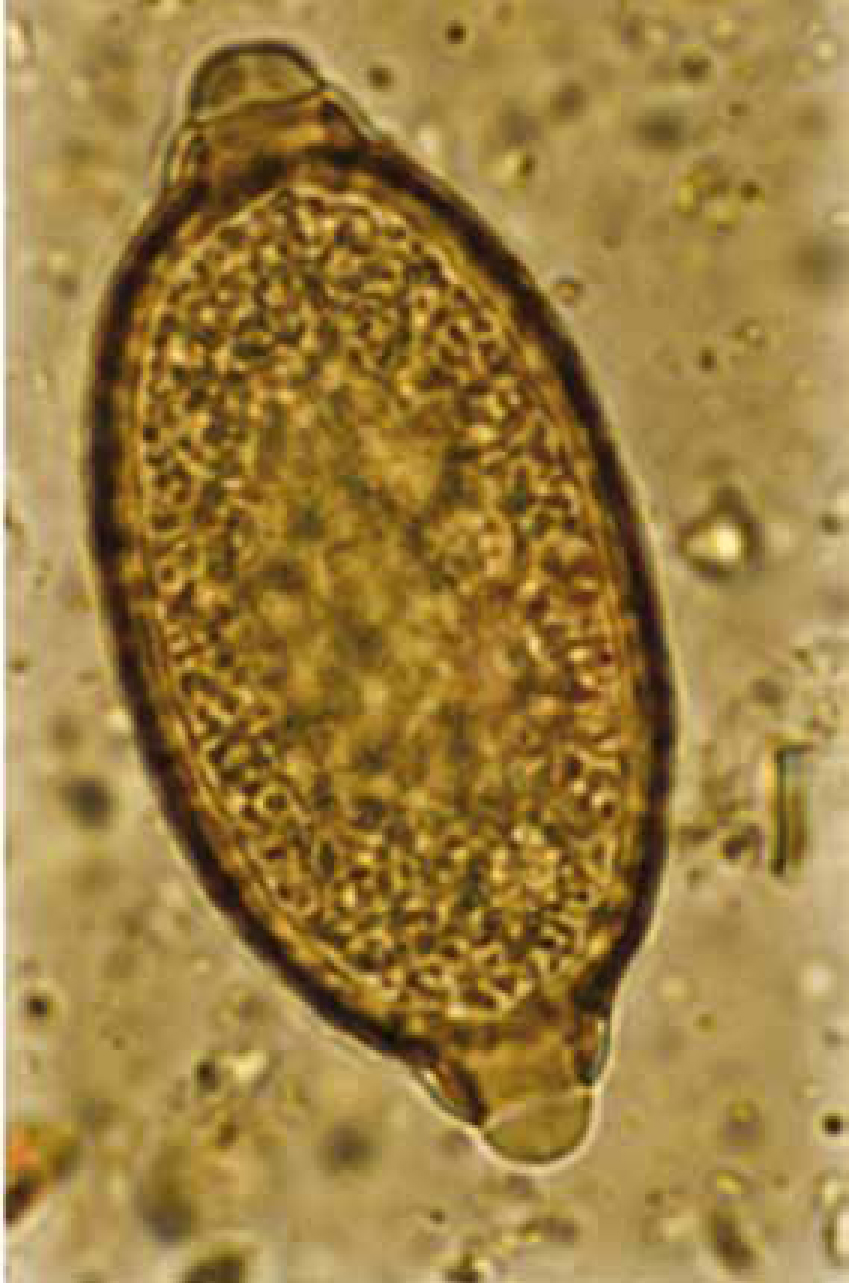
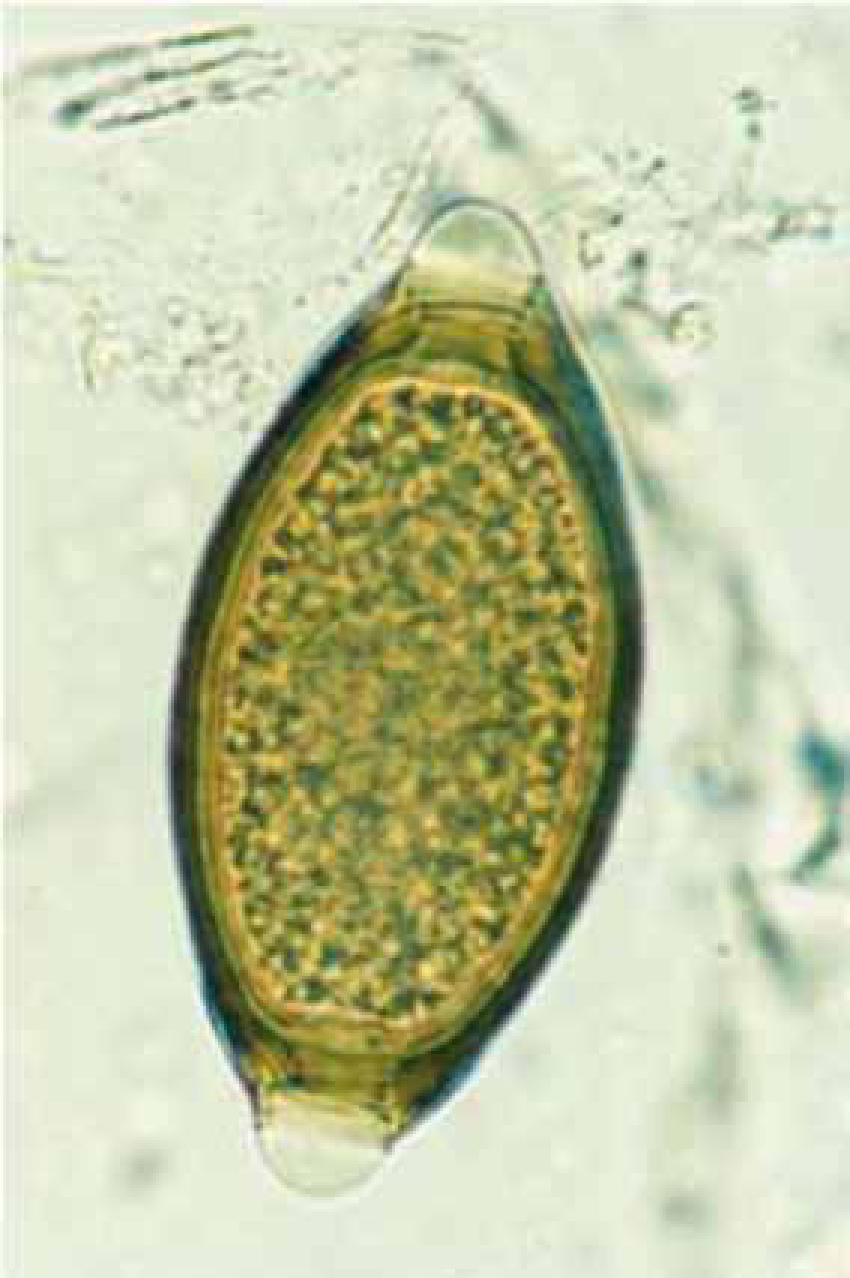
Eucoleus aerophilus (=Capillaria aerophila; Superfamily Trichuroidea) is another rare canine lungworm that produces eggs, not larvae, which may be found in both faeces and BAL (Burgess et al, 2008). A recent survey in Italy suggests that this nematode is an emerging, clinically important entity of cats and dogs (Traversa et al, 2009), and is an underestimated problem in Europe (Traversa et al, 2010). In December 2010, the authors identified E. aerophilus eggs in a faecal sample obtained post mortem from a 2-year-old dog in Shropshire, the sample had been submitted for routine screening faecal parasitology. Numerous eggs of E. aerophilus were detected and identified according to the descriptions of Schoning et al (1993). E. aerophilus is a very common lungworm of foxes in the UK (Morgan et al, 2008) but this appears to be the first report of E. aerophilus from a dog in the UK. A differential diagnosis from the relatively common large intestinal nematode, Trichuris vulpis, is the main issue here: the eggs are very similar and may be separated by examining the shell and relative position of the polar plugs. In E. aerophilus, the surface of the shell is striated around the plugs with a general surface corrugation, and the plugs are asymmetrically opposed (Figure 5). The shell surface of T vulpis is smooth (Figure 6) and the plugs are positioned directly opposite each other.
Concentration techniques
The reliable use of solutions such as saturated sodium chloride to float L1 has been questioned. In order to float larvae, higher density solutions are needed, but can distort larval morphology, making identification difficult (Traversa and Guglielmini, 2008). The findings of case one appear to contradict this, and the extent to which larvae become distorted in any concentrated salt solution will depend on the type of salt, the specific gravity and length of time left in contact. This was demonstrated many years ago by Georgi et al (1977), in his work on Filaroides hirthi in the USA, when he found that although L1 became distorted in sucrose (specific gravity not given) they could retain their shape, motility and viability in zinc sulphate (specific gravity 1.18) for 1–2 hrs. Flotation of L1 in zinc sulphate at that specific gravity proved to be approximately 100 times more efficient than a Baermann technique in concentrating larvae from dog faeces.
In recent years commercially developed concentration techniques for parasite eggs using variations on salt flotation methods have become more popular in medical and veterinary laboratories alike. The FLOTACR technique (Cringoli, 2006) is a relatively new method used to quantify intestinal worm eggs and cysts in animal faecal samples. When evaluated for the detection of the lungworm Crenosoma vulpis the technique was found to be more sensitive than either salt flotation or Baermann sedimentation (Rinaldi et al, 2007). This technique may prove useful for other dog lungworm larvae especially when faecal samples have been stored, which reduces larval viability, thus excluding the use of larval migration methods (Schnyder et al, 2010). However, this method requires special equipment that is unlikely to be available in practice laboratories. A comparative evaluation of performance against current methods is required for faecal concentrators such ParasepR tubes (www.diasys.com/ (Wokingham, Berkshire) with a built-in sieve of appropriate size. A centrifugation step produces a small amount of sediment for clean examination, and the system is enclosed with the operator protected from possible zoonotic agents.
In house testing
Although more sensitive coprological tests (faecal examinations) to detect lungworm larvae would be useful, and should be evaluated, existing technology is under used at practice level. Indeed, there is a case that faecal tests, to include recovery of larvae as well as eggs, should be conducted routinely in cases of respiratory disease in dogs in the UK, whether in house or by external laboratories. Faecal tests have the advantage that they are relatively cheap and easy to conduct, and are broad in spectrum. Regular use of coprological diagnosis can greatly help to build up a picture of which parasites are a common cause of disease in the catchment area of a particular practice, and therefore guide practice protocols for diagnosis, treatment and preventative worming. This is especially relevant given expanding but apparently patchy occurrence of A. vasorum, as well as the possible underestimation of other lungworms, on which little epidemiological information is available.
Conclusions
Given the potential and rapid onset and severity of lungworm disease, a rapid and reliable definitive diagnosis is essential and decreases unnecessary investigation. Coprological approaches continue to have a role in clinical and epidemiological investigations, and could be further improved. This could include an evaluation of different concentrated salt solutions — saturated sodium chloride, sucrose and magnesium chloride and others — traditionally used in parasitology laboratories for the eggs of intestinal worms.
