Haemophilia A is a severe, inherited, bleeding disorder that affects several mammals including dogs (Aslanian et al, 2014). It has an estimated incidence in human populations of 1–2 cases per 10 000 male births (Brooks et al, 2005). However there are fewer reported studies in veterinary medicine and the prevalence of dogs with haemophilia A is unknown. Similar to the human condition, it is associated with high morbidity and mortality (Callan et al, 2016). It is caused by a deficiency of clotting factor eight, represented by Roman numeral VIII or (FVIII) which is vital for blood clot formation (O'Kelley et al, 2009). Haemophilia A is the result of a spontaneous gene mutation in FVIII, preventing the body from clotting normally. It is diagnosed by a combination of prolonged coagulation times together with a reduction in FVIII activities/concentrations (Dunning et al, 2009).
This report discusses an individual case that presented to the hospital for lameness and was subsequently diagnosed with haemophilia A. It also provides an overview of haemophilia A including clinical signs, diagnosis and treatment of the disorder. Orpet and Jeffery's Ability Model (2007) was used as a reference to carry out the nursing assessment, from the time of admission until discharge, which provided an ideal framework for which to prioritise nursing requirements (Orpet and Jeffery, 2006).
Case history and clinical findings
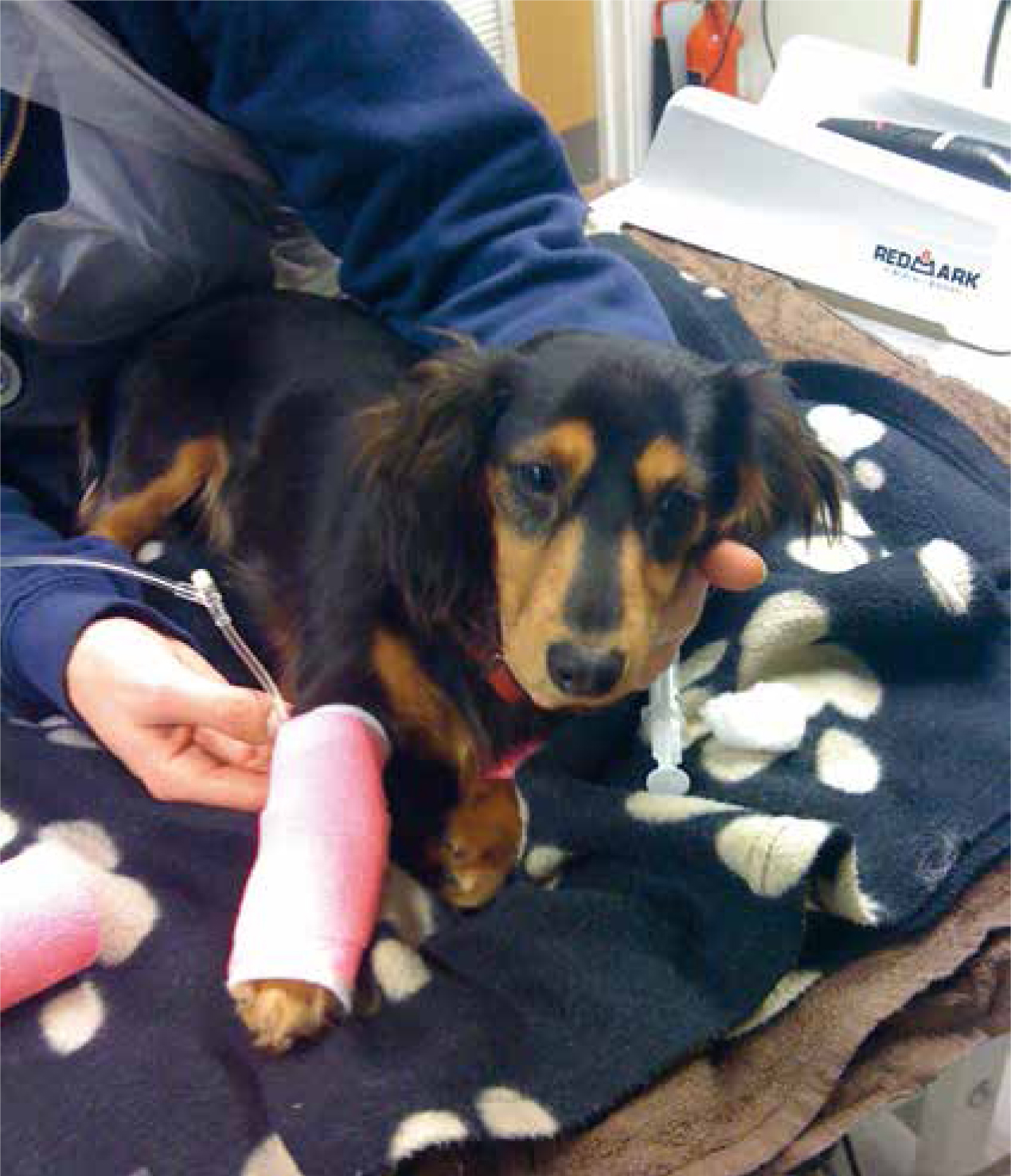
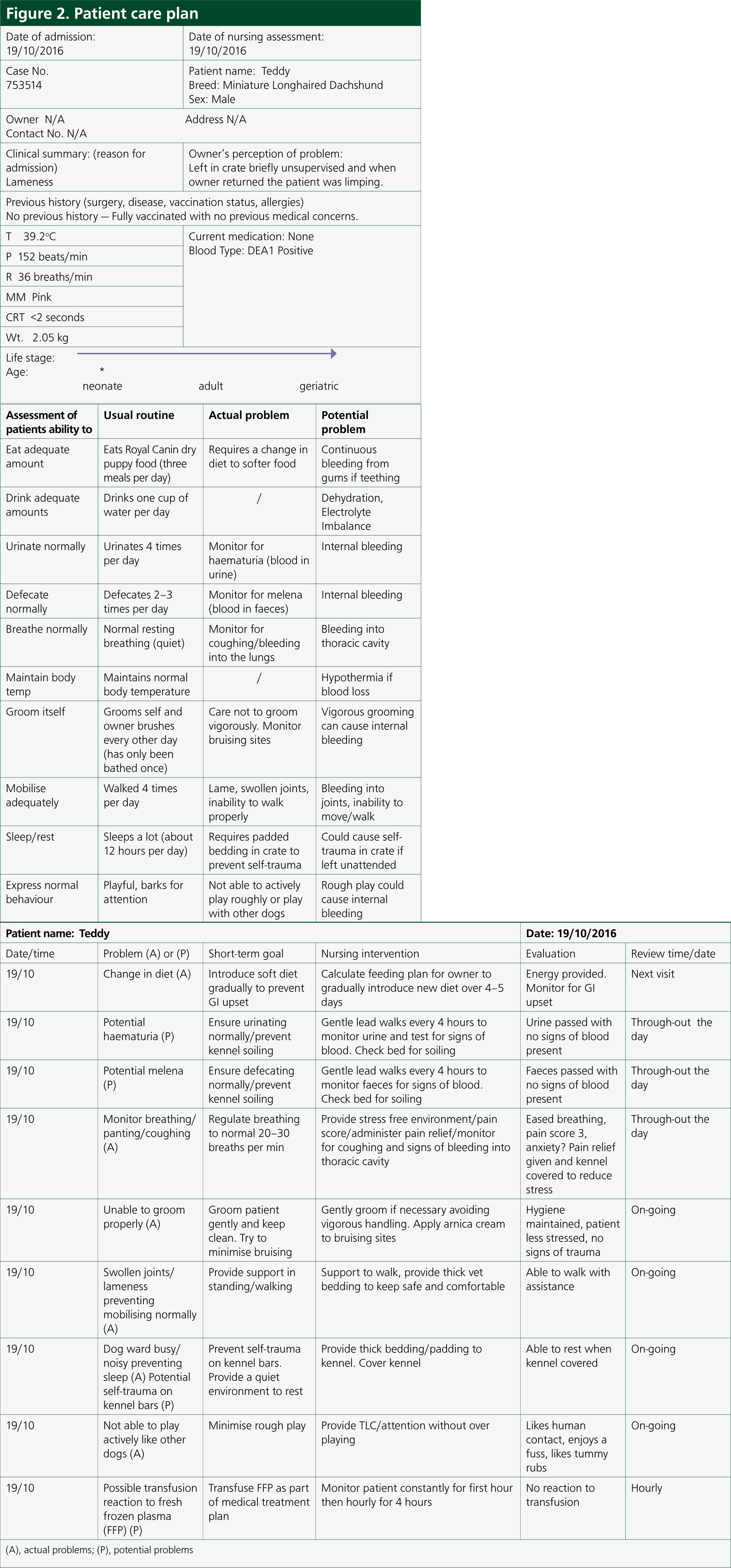
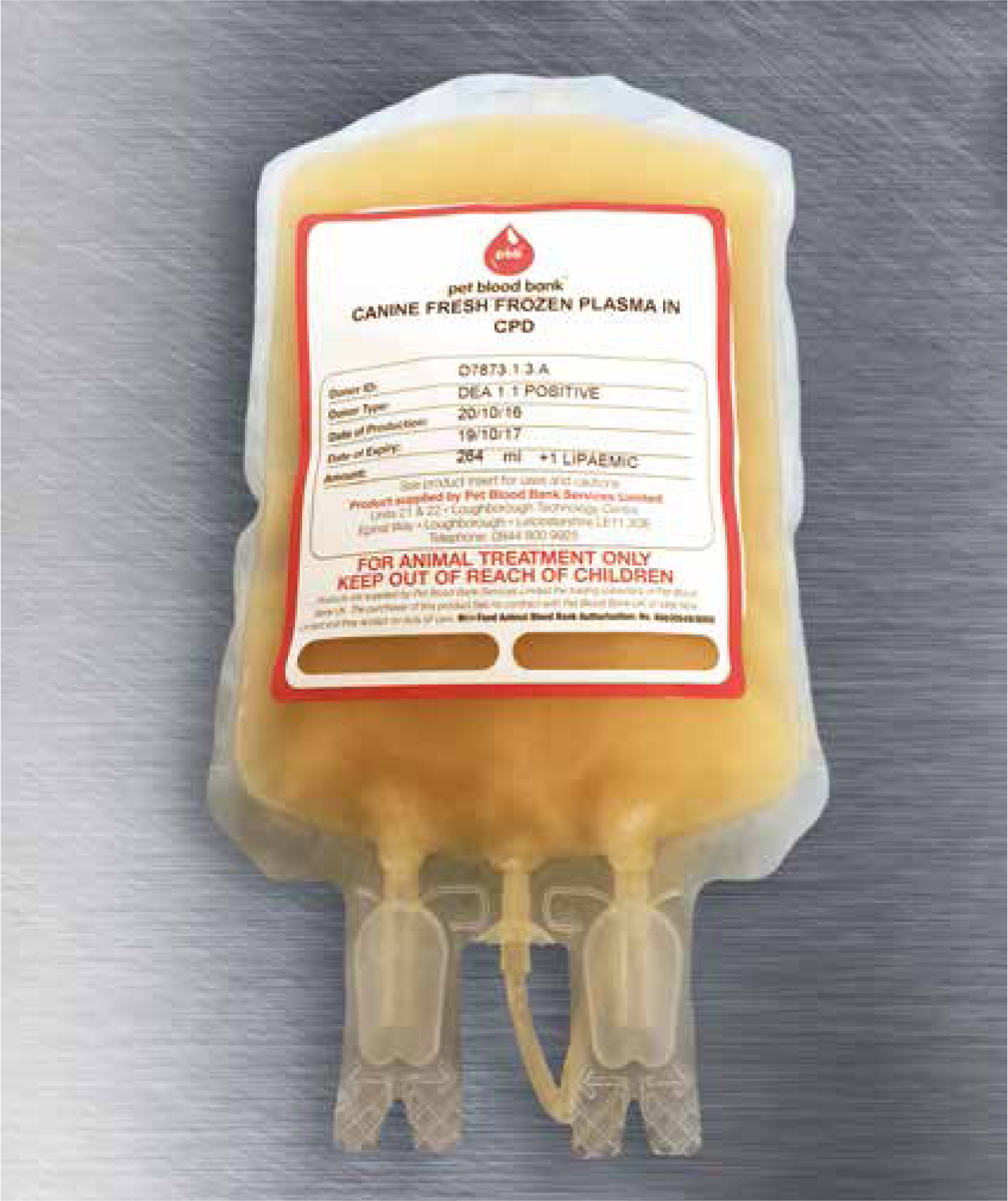
A 6-month-old, male entire Miniature Longhaired Dachshund (Figure 1) presented due to acute onset lameness to the left forelimb. The patient was approximately 7/10 lameness with pink mucous membranes, capillary refill time of <2 seconds; heart rate was 152 beats per minute with strong, synchronous pulses. A soft tissue injury was suspected as the limb was tender on manipulation. The patient was given a subcutaneous injection of meloxicam at 0.2 mg/kg loading dose and subsequently sent home to rest with oral meloxicam at a dose of 0.1 mg/kg every 24 hours together with tramadol at a dose of 2 mg/kg three times per day. However the patient re-presented on a further three occasions for lameness and joint swelling of various limbs. Survey radiographs, of all four extremities, were then carried out which involved lateral views of both forelimbs (to include the humerus down to the digits) as well as lateral views of both hindlimbs (to include the femur down to the digits). This was performed to rule out any obvious bone pathology, e.g. fractures, panosteitis or metaphyseal osteopathy. Radiographs performed revealed soft tissue swelling of the joints. The patient also developed a large soft tissue swelling at the site of microchip implantation. Meloxicam was used sporadically to manage the intermittent lameness. It was then decided to refer the dog for further investigations. On presentation to an internal medicine specialist, the soft tissue swelling between the scapulae was still present and there was swelling of both carpal joints. As a veterinary nurse, it was important to also gain as much information about the patient's usual routine as well as clinical history from the owner in order to provide the necessary nursing care (see Figure 2 for the full patient care plan). Laboratory work up consisted of blood samples taken to measure complete blood counts, packed cell volume/total solids, blood smear cytology and clotting times. In-house blood samples were taken from the patient's peripheral cephalic vein, as jugular samples should be avoided in patients with suspected coagulopathy. Rose et al (2013) recommend a manual blood smear to count platelets as well as laboratory testing, which were carried out. The patient revealed moderate anaemia showing signs of regeneration, likely to be secondary to blood loss with a packed cell volume of 28% and a total solid value of 62 g/litre. A complete blood count revealed normal platelet numbers which was also confirmed by examination of a manual blood smear under the microscope. Clotting time blood tests were then performed in-house which included activated partial thromboplastin time (APTT) and prothrombin time (PT). APTT was significantly prolonged, with a value of 154 (reference range: 72–102), while PT was just below the normal range at 9 (11–17). The patient received a transfusion of canine fresh frozen plasma (FFP) (Figure 3) given at a rate of 10 ml/kg over 4 hours, where no adverse reactions were noted during or immediately after transfusion. In-house clotting values were retested post transfusion with an improvement noted in APTT times. Due to the abnormal clotting times, further investigations were carried out to look at individual clotting factors. The factors measured were XII, XI, IX and VIII. Results were pending for a few weeks. On discharge, the patient was prescribed tranexamic acid, at a dose of 62.5 mg (1/8 of a 500 mg tablet) twice daily for 14 days together with a 5 day course of tramadol 10 mg (5 mg to be given twice daily). A re-visit appointment was made for 2 weeks where the results revealed a diagnosis of moderate haemophilia A. There was a severe reduction in the patient's FVIII activity, compatible with a diagnosis of haemophilia A (FVIII deficiency).
Haemophila A
Discussion
The gene for FVIII is carried on the X chromosome and is known as sex-linked inheritance (Littlewood, 2012). Having one normal gene is enough to prevent haemophilia A so the condition can be described as X-linked recessive, predominantly affecting males with females as carriers (Gavazza et al, 2014). Males inherit one gene for FVIII whereas females have two genes for FVIII, as one is inherited from their mother, and one from their father. Males that have a normal X gene and females that have two normal X genes are entirely free from haemophilia A and cannot transfer this to their offspring. Males that have an abnormal X gene are affected with haemophilia A and will transmit this to all of their daughters. Females that have one normal X gene and one abnormal gene are known as asymptomatic carriers (heterozygous) and will transmit the abnormal gene to 50% of their sons and 50% of their daughters. Looking at the X chromosome study conducted by Cornell University College of Veterinary Medicine (2014), if the normal gene for FVIII is represented as XH and the abnormal or haemophilic gene is Xh then the different possibilities for clinical inherited information (phenotype) and genetic characteristics (genotype) can be seen in Table 1; Table 2 categorises the type of offspring produced with an asymptomatic carrier female and a normal male:
Table 1. X chromosome study phenotype and genotype of the haemophilic gene
| Phenotype | Genotype |
|---|---|
| Normal male | XHY |
| Normal female | XHXH |
| Affected male | XhY |
| Carrier female | XhXH |
| Affected female | XhXh |
Table 2. The type of offspring produced with an asymptomatic carrier female and a normal male
| Parent type | Offspring type | Genotype |
|---|---|---|
| Carrier female & Normal male (XhXH & XHY) | Normal male | XHY |
| Affected male | XhY | |
| Normal female | XHXH | |
| Carrier female | XhXH |
FVIII deficiency can affect many pure and crossbreed dogs as well as various cats. Cases of haemophilia A have been reported in the UK, Australia as well as North America (Brooks et al, 2005). Patients tend to have normal PT times and prolonged APTT times (Littlewood, 2012). Several studies have shown that the severity of haemophilia A correlates inversely to the specific measurement of FVIII coagulant activity (Brooks et al, 2005; Callan et al, 2016).
Clinical signs
Affected puppies born with haemophilia A can have prolonged bleeding from the umbilical cord straight after birth. They also develop bleeding from the gums during teething and prolonged bleeding after an injury or surgery. Further clinical signs include lameness due to spontaneous bleeding into joints, sudden clot formation that can cause subcutaneous haematomas, intermittent bleeding at injection sites and fatal bleeding into the body cavity (thorax or abdomen) (Aslanian et al, 2014). Severe cases result in death within the first few weeks of life. Less severe cases will survive but will experience recurrent bleeding.
Diagnosis
The diagnosis is harder to confirm in patients less than 6 months old due to the possibility that their liver is not producing enough of the clotting proteins (Callan et al, 2016). A citrated plasma blood sample is required to test and run a coagulation assay. The APTT is a screening test that can be carried out in-house to detect coagulation defects. This test will be abnormal in haemophiliacs. In addition to this, a specific measurement of FVIII coagulation must be carried out as dogs with haemophilia A will have a reduced level of this factor compared with normal dogs (Gerber et al, 1999).
Management and treatment
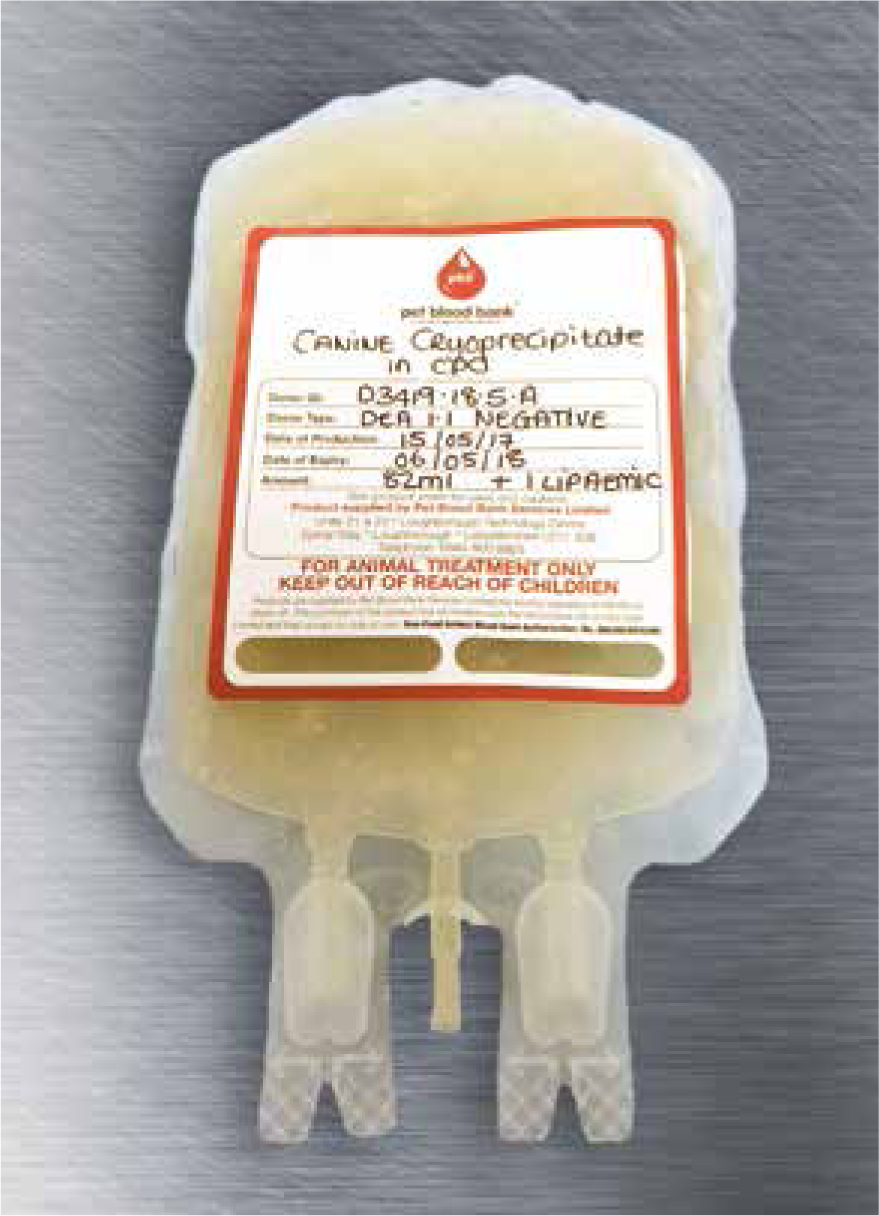
Treatment of haemophilia A requires replacement of the missing FVIII which can be achieved through repeated transfusions of whole blood, fresh frozen plasma (FFP) or cryoprecipitate (O'Kelley et al, 2009) until the bleeding has been controlled. However alloantibodies that neutralise the protein replacement therapy develop in 20–30% of patients with moderate to severe haemophilia A, making certain patients very difficult to treat. Recommended guidelines state 1 unit/10 kg of either plasma or cryoprecipitate (Figure 4) can be given every 4–12 hours (as required) with a transfusion rate of 1–2 ml/minute over 4–6 hours. The Pet Blood Bank was the source of FFP given in this case. Cryoprecipitate has the advantage of providing FVIII at a lower volume than FFP and it also contains fewer plasma proteins that can cause a transfusion reaction (Stokol and Parry, 1998; Aslanian et al, 2014).
An alternative to transfusion for acute haemophilia A is desmopressin I-desamino-8-arginine vasopressin (DDAVP) (Figure 5), which is a synthetic analogue of vasopressin that increases levels of FVIII (Mathews et al, 2006). It releases body stores of the factor and has been used successfully to treat humans with haemophilia A (Stokol and Parry, 1998). As FVIII is found circulating in plasma, DDAVP increases plasma concentrations of FVIII and can be administered intravenously or subcutaneously (Mansell and Parry, 1991). Studies have shown that dogs do not respond as well to DDAVP administration as humans, therefore it should not be relied on solely as treatment to improve the patient's clotting process (Mansell and Parry, 1991).

Borchers (2015) describes tranexamic acid (Figure 6) as another treatment option as it helps prolong activated thrombin times at a high enough dose. It is a medication used to treat or prevent excessive blood loss and is an antifibrinolytic which works by inhibiting plasmin from binding with fibrin (Borchers, 2015). A human trial found that tranexamic acid reduced mortality in trauma patients with significant bleeding, if given early enough (Borchers, 2015). Long-term tranexamic acid suspension was reconstituted for this patient.
Despite the ready access to plasma products in veterinary medicine, Margaritis et al (2009) successfully treated a canine haemophilia A patient using gene therapy as used as an alternative treatment. Connelly et al (1996) also carried out a study to see if gene therapy could be an alternative treatment for haemophilia A and found that effective treatment of canine haemophilia A was achieved with a peripheral vein administration of a human FVIII adenoviral vector. Further advances in vector design are required, however this could be an alternative for the correction of the coagulation defect. Finn et al (2010) conducted a successful study of a liver gene expression to neutralise antibodies to FVIII in order to treat haemophilia A. Another study, whereby a platelet gene transfer, encoding FVIII, was also trialled by Du et al (2013) as a successful alternative to treatment of haemophilia A. This study showed that there was a prevention of severe bleeding episodes in dogs with haemophilia A for at least 2.5 years after transplantation (Du et al, 2013). Callan et al (2016) first reported successful gene therapy in dogs with haemophilia A resulting in significant improvements aiding to the success rate of an alternative treatment to the condition.
Process of nursing
Orpet and Jeffery's Ability Model (2007) was used to carry out the nursing assessment, using an ideal framework from which to prioritise nursing requirements, as it has been adapted to care specifically for veterinary patients, where the work of Roper, Logan and Tierney's Model-Activities of Living and Orem's Model of Self Care influenced the creation of this model (Nelson and Welsh, 2015). Using this model, and by following the questionnaire structure, an initial assessment of the patient was made following the ten abilities of the animal. An important question to ask was the patient's normal routine to include the type of food eaten and at what times, normal toileting routine (outside/on grass) and a description of normal behaviour. Once the patient was admitted, it was then important to work out what the patient could and could not do. Carrying out the initial assessment stage highlights problems that the patient is experiencing, as well as potential problems that may arise, and that must be prevented. Thus a decision can be made on the nursing care required.
Using Orpet and Jeffery's Ability Model (2007), several problems were identified and the necessary nursing interventions put in place in order to care for the patient. According to Leenerts et al (1996) a care plan is important as it highlights exactly what the nursing interventions are and how often the care is required. These decisions are attached very closely to the goals to be achieved while providing nursing care, which, in turn, measures the outcome of the nursing intervention. A number of problems were identified with the patient post admission, and a list of nursing interventions were discussed and then implemented throughout the course of the day.
Due to closely following Orpet and Jeffery's Ability Model (2007), a systematic and careful analysis of the patient care required was provided, and ensured nursing interventions were conducted to provide the best possible care for the patient.
Recommendations and outcome
Patients diagnosed with haemophilia A require a change in lifestyle, and in this case, the patient had to make several alterations to his everyday life in order to reduce significant bumps/trauma. Padded bedding and a padded cage was required to prevent self-trauma on the bars when left unattended. Daily checks for swelling, bruising and bleeding were also necessary. Exercise restrictions were recommended as this may limit joint bleeds and lameness. Tranexamic acid was the long-term medication used to manage the disorder however side effects of this medication have been shown to cause hypotension, nausea, vomiting, diarrhoea and abdominal cramps (Borchers, 2015). A study conducted by Aslanian et al (2014) suggested that there is an acceptable long-term outcome for haemophilia A patients and that prognosis should not solely be determined by FVIII deficiency.
Further close communication between the client and veterinary surgeon/veterinary nurse is necessary for treating a patient with haemophilia A as financial limitations may prevent successful management of the disorder. Counselling is also recommended to the client with emphasis on financial constraints and lifestyle changes in treatment of the condition. It is important to explain that current treatments for haemophilia are not curative however the disease can be manageable. Males that are affected by moderate haemophilia A (like this case) often survive to adulthood (Brookes et al, 2005). Conditions such as haemophilia A can be more easily treated than previously due to the emergence of veterinary pet blood banks and increased use of blood products allowing multiple transfusions in a single patient. With any medical disorder, nursing models are used because they provide consistency in the care given to the patient, there is less conflict between nurses on the care required, it incorporates the care given by other members of the team, it gives direction in nursing care and guides decision making (Orpet and Jeffery, 2006), thus should be used in every case.
Applying Orpet and Jeffery's ability model enabled the veterinary nurse involved to build up a relationship with the patient as well as the owners. It allowed her to highlight problems in the patient's daily activities, and provide specific nursing interventions to assist the patient. Not only did it allow her to provide nursing care to the patient, but it also led to improvements being made to enable the patient to continue to live a healthy, manageable and pain free life. The model demonstrated the importance of activities and the impact it has on the patient if they cannot perform any of these activities.
Conclusion
Haemophilia A patients experience spontaneous bleeding into joints and soft tissues, and when left untreated, the bleeding can be fatal. In this case, the treatment for the bleeding disorder was focused on the use of FFP for acute response. In addition, a more long-term conservative treatment consisted of tranexamic acid together with pain relief, which resulted in a fair response in reducing the spontaneous bleeding. The patient however, deteriorated over the course of 6 months and due to poor quality of life, unfortunately was euthanised.
Key Points
- Haemophilia A is a severe, inherited, bleeding disorder and is caused by a deficiency of clotting factor eight, represented by Roman numeral VIII or (FVIII) which is vital for blood clot formation.
- Haemophilia A patients experience spontaneous bleeding into joints and soft tissues, and when left untreated, the bleeding can be fatal thus continual monitoring is vital.
- Clinical signs of haemophilia A include lameness due to spontaneous bleeding into a joint, sudden clot formation that can cause subcutaneous haematomas, intermittent bleeding at injection sites and fatal bleeding into the body cavity (thorax or abdomen).
- Treatment of haemophilia A requires replacement of the missing FVIII which can be achieved through repeated transfusions of whole blood, FFP or cryoprecipitate.
- Patients diagnosed with haemophilia A require a change in lifestyle.
- Supportive nursing care (following Orpet and Jeffery's Ability Model (2007)), allowed a systematic and careful analysis of the patient's required needs, thus ensuring nursing interventions were conducted to provide the best possible care for the patient.


