Despite the recent increase in the popularity of pet rabbits and the dramatic increase in their presentation for treatment in the veterinary surgery (Eatwell and Mancinelli, 2013), according to research studies they are at a higher risk of perioperative mortality than both canines and felines (Brodbelt et al, 2008). In the last 20 years there have been extensive publications regarding the care of hospitalised rabbits, it is important for the veterinary nurse to access the information available and to critically evaluate the conclusions made; this would allow the improvement of patient care through the use of the most up-to-date, evidence-based nursing care (Bloor, 2012). In order to reduce the instances of mortality in hospitalised rabbits, the veterinary nurse must develop an understanding of the factors commonly associated with this outcome and take preventative measures.
Rabbits are a prey species that, in the wild, are the target of many predators resulting in them demonstrating ‘flight’ behaviour in stressful situations. Rabbits are catecholamine-driven animals and are very susceptible to stress, which in extreme cases, can result in cardiac arrest. Due to the predation they experience rabbits are adept at concealing signs of illness to prevent appearing weak to predators. This poses problems, as signs of illness are well hidden and without astute veterinary professionals serious illness can go undetected. On presentation to the veterinary surgery, many rabbits are in a critical state of health, often due to a combination of their ability to conceal illness and inexperienced ownership (Mancinelli, 2015a).
Rabbits are rapidly increasing in popularity and Statista (2016) reported that they are the third most popular pet owned in the UK; their presentation at the veterinary practice is increasingly common and owners expect standards of care equal to that provided for other species. Despite this, little training is provided to veterinary professionals. For example, few colleges teach veterinary surgeons to perform rabbit ovariohysterectomies (Lewis, 2010), placing rabbits at increased risk during these procedures. This makes the undertaking of continued professional development essential to improve knowledge relating to this species and therefore the care provided; it is now possible to undertake qualifications in exotic pet medicine and care. If these opportunities are utilised veterinary nurses will benefit from a better understanding of this species and their requirements when hospitalised.
Anaesthesia
There is a general consensus among veterinary professionals that rabbits are at a greater risk of mortality than canines or felines when hospitalised for veterinary treatment (Lewis, 2010; Wenger, 2012; Eatwell and Mancinelli, 2013, Druce, 2015). This is a potentially harmful opinion to hold; if a patient is assumed to be at a higher risk, fatalities may be attributed to this, rather than evaluating the care provided to determine shortfalls. In a 2008 study by Brodbelt et al it was determined that, in relation to anaesthesia, healthy rabbits had a 0.73% risk of mortality, as opposed to the canine and feline risk of 0.05% and 0.11% respectively. This percentage increased significantly for sick rabbits to 7.37%, whereas the increase for canines and felines was significantly less at 1.33% and 1.40% respectively. Despite the fact that the study used a larger sample size for both canines and felines than rabbits, it was concluded that the risk to both healthy and sick rabbits is greater. This could be a result of underlying conditions that are not evident due to the rabbit's ability to conceal illness, meaning patients present in an advanced stage of illness, or as a result of patient care. Both canine and feline patients are not able to hide signs of illness as effectively as rabbits and often live inside the house with the owner, where subtle signs can be noticed with ease resulting in early presentation at the veterinary practice. In contrast, many rabbits are housed outside and are not observed for a large portion of the day, meaning subtle signs can easily be missed. As the confidence intervals for the study were 95% it is likely that the results obtained are accurate. As a result of this study, it has been recommended that rabbits, particularly those in poor health, should be stabilised prior to being anaesthetised (Longley, 2009); stabilisation can be achieved through the administration of fluid therapy, nutritional support, analgesia and pro-kinetics. Rabbits have a higher maintenance requirement for fluids than cats and dogs and, therefore, the correction of hypovolaemia and electrolyte imbalances is essential to stabilise the patient before any surgical intervention can take place (Hedley, 2011). Provision of analgesia is vital to prevent the disturbance of gastrointestinal function that occurs as a result of unmanaged pain. Preparation is key in rabbit anaesthesia.
The sensitivity of the rabbit's respiratory system can result in many anaesthetic drugs having a low therapeutic index (Borkowski and Karas, 1999). Veterinary nurses must understand the implications associated with the anaesthetic drugs administered in order to perform appropriate monitoring of patients while anaesthetised; for this reason it is essential to ensure that each practice has a clear anaesthetic protocol for rabbits.
Breath holding is commonly reported in rabbits anaesthetised with inhalation agents, and this apnoea can result in the patient developing hypoxaemia (Wenger, 2012), which can result in ischaemia to highly oxygen dependant tissues, such as the brain (Jones and Berry, 2015). It is important to consider this before choosing an anaesthetic protocol; regardless of the anaesthetic protocol used all patients should be pre-oxygenated before induction and respiration monitored carefully.
The position of the patient while under anaesthesia can affect the quality of respiration (Woolfe, 2012). Rabbits have a large abdominal visceral volume which can increase pressure on the diaphragm, therefore these patients are at increased risk of respiratory compromise without appropriate positioning (Harcourt-Brown, 2005a; Longley, 2008). The patient should be supported with the thorax elevated above the abdomen, this can be achieved by tilting of the theatre table or supporting the patient with towels (Figure 1).
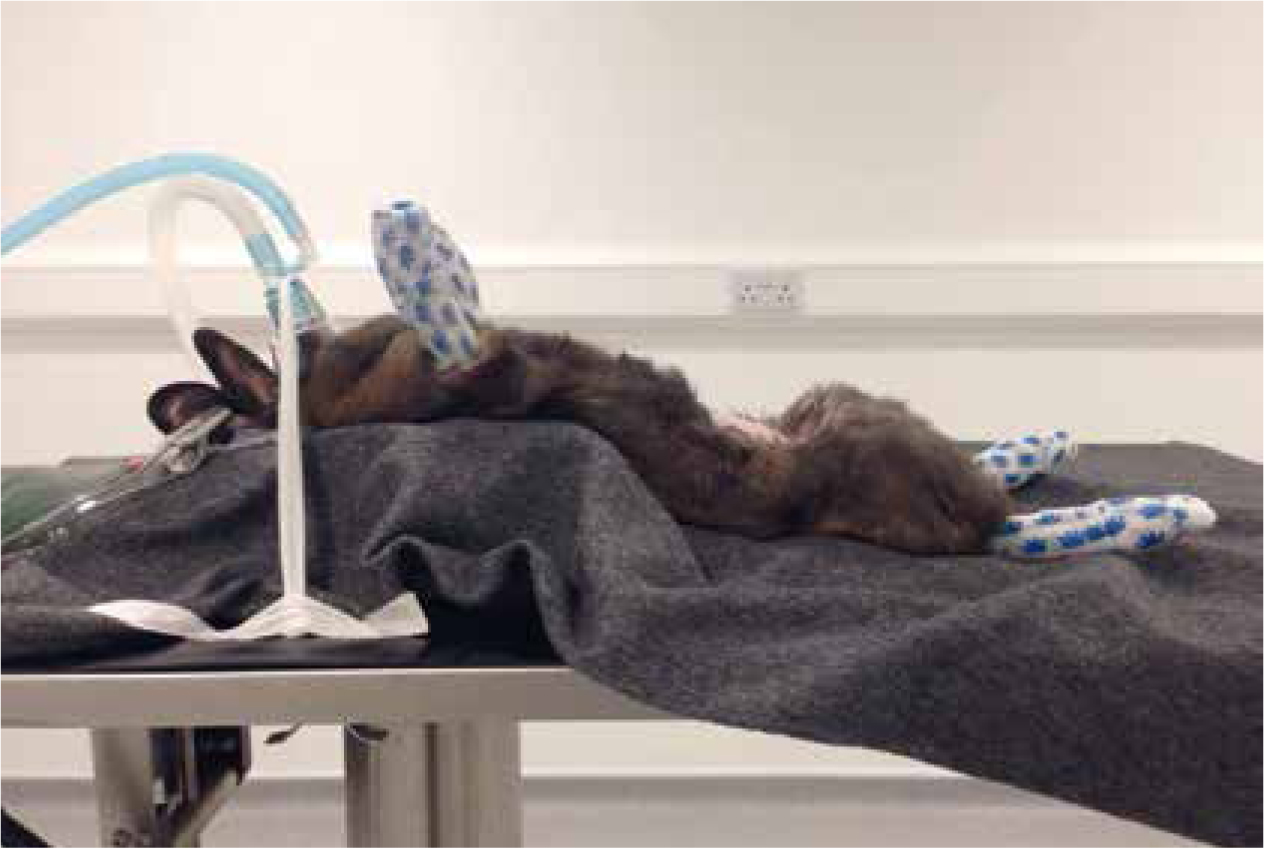
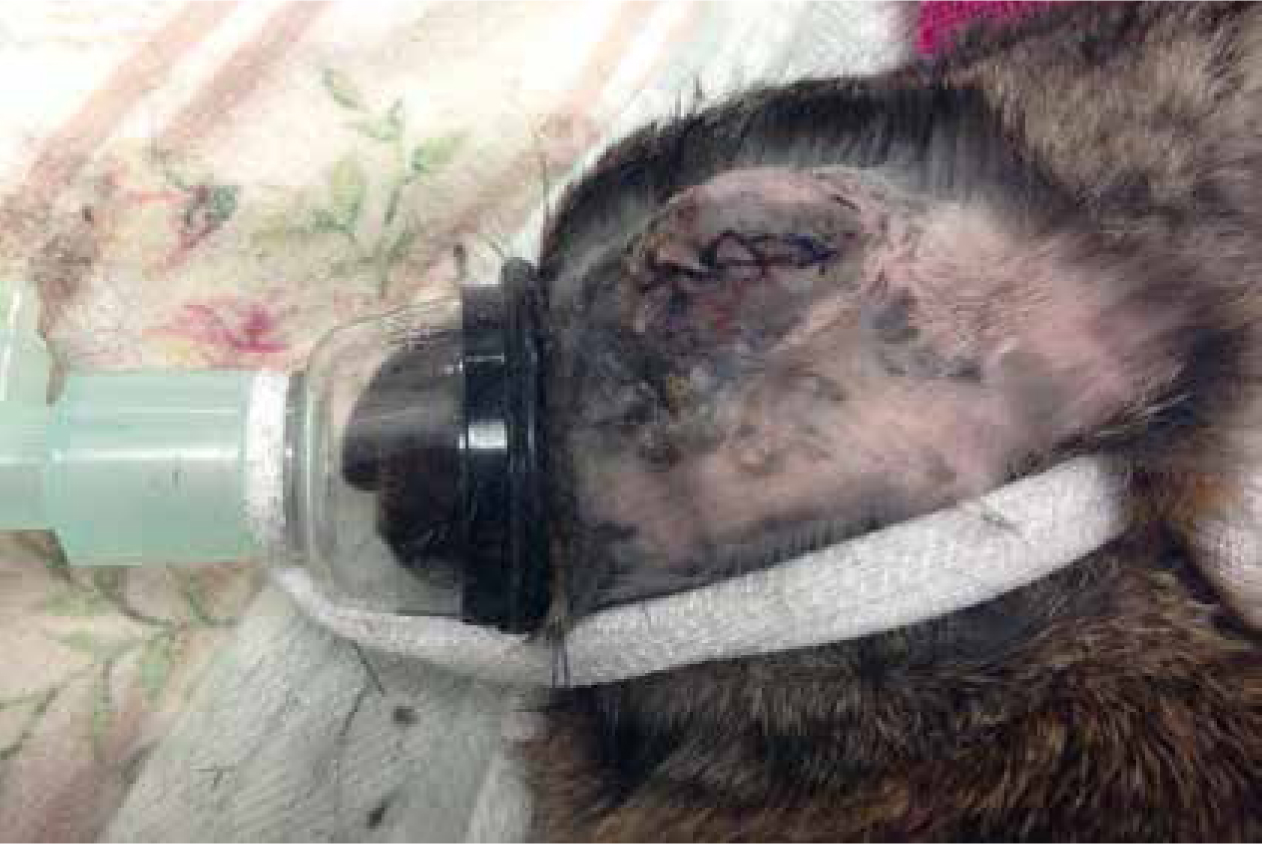
There are various opinions regarding airway management in rabbits; Eatwell and Mancinelli (2013) determined that only 29% of rabbits are intubated in practice. Airway management can be achieved by endotracheal intubation, face masks or the use of supraglottic airway devices. There are risks associated with the incorrect intubation of rabbits (Eatwell and Mancinelli, 2013); complications of improper placement can include tracheal trauma and oedema, which can impair respiration or lead to respiratory arrest. Complications can occur if the patient is not sufficiently anaesthetised or if the tube is placed by inexperienced personnel; for this reason, intubation should only be performed by those suitably trained. A tightly fitting, small mask that covers both nares is an appropriate method of airway management where intubation or the use of a supraglottic airway device is not possible (Lewis, 2010) (Figure 2). Lawton (2005) recommends securing the patients airway to allow intermittent positive pressure ventilation (IPPV) if respiratory arrest occurred during the anaesthesia. The use of supraglottic airway devices can be beneficial for rabbit anaesthesia, as they can be placed with ease and reduce the length and risk of intubation (Wenger, 2012; Eatwell and Mancinelli, 2013).
Buckley et al (2011) suggested that the instance of cardiopulmonary arrest is common in rabbits in the hospital environment; while the research performed suggested a correlation between anaesthesia and mortality, it did not determine a specific point in the anaesthesia where the patient was at most risk. The results of this research may also be questioned due to the small sample size and therefore the potential for unrepresentative results. A number of studies determined that the postoperative period posed the highest risk for mortality in small mammals (Brodbelt et al, 2008; Wenger, 2012). While the study identified that this period was the highest risk for all species, rabbits were shown to exhibit higher rates of postoperative mortality than the other species studied. This indicates that patients should be monitored as closely in the postoperative period as they are intra-operatively to ensure a good patient outcome. In order to allow easy monitoring and access, patients can be recovered in a padded, wire basket in the prep area until both the nurse and veterinary surgeon are happy for them to be returned to the wards (Figure 3). Postoperatively, heart rate, respiration rate, temperature and pain should all be monitored regularly, and once the patient is adequately conscious, food and water can be offered, and intake recorded (Table 1).

| Heart rate: | 180–350 beats per minute (bpm) | |
|---|---|---|
| Respiration rate: | 30–60 bpm | |
| Temperature: | 38.6–40.1°C | |
| Capillary refill time (CRT): | ~2 s | |
| Blood pressure: | Mean arterial pressure: | 80–91 mmHg |
| Systolic blood pressure: | 92.7–135 mmHg | |
| Diastolic blood pressure: | 64–75 mmHg | |
| Packed cell volume (PCV): | 33–50% | |
| Blood glucose: | 5.5–8.2 mmol/litre | |
| Urine volume: | 10–35 ml/kg/day | |
| Specific gravity: | 1.003–1.036 | |
| Fluid requirements: | Maintenance: | 80–100 ml/kg/day |
| Deficits: | 10 ml/kg per 1% dehydration | |
Temperature
The occurrence of hypothermia should be prevented in hospitalised rabbits (King, 2009; Wenger, 2012). Hypothermia can result in circulatory and respiratory collapse, prolonged recovery periods and inappetence, all of which can contribute to increased mortality rates (Druce, 2015). Rabbits are predisposed to the development of hypothermia due to their high surface area to volume ratio (Brodbelt et al, 2008), therefore, these patients should be actively heated to maintain normothermia until fully recovered (Wenger, 2012). Vigilant nursing care is required during the recovery of the patient to ensure that hyperthermia is not induced, the effects of which can be equally as detrimental to the patient as hypothermia (Pei et al, 2012). Rabbits are intolerant to heat and a paediatric human incubator can be used so that the temperature can be adjusted as the patient recovers (Edis et al, 2016).
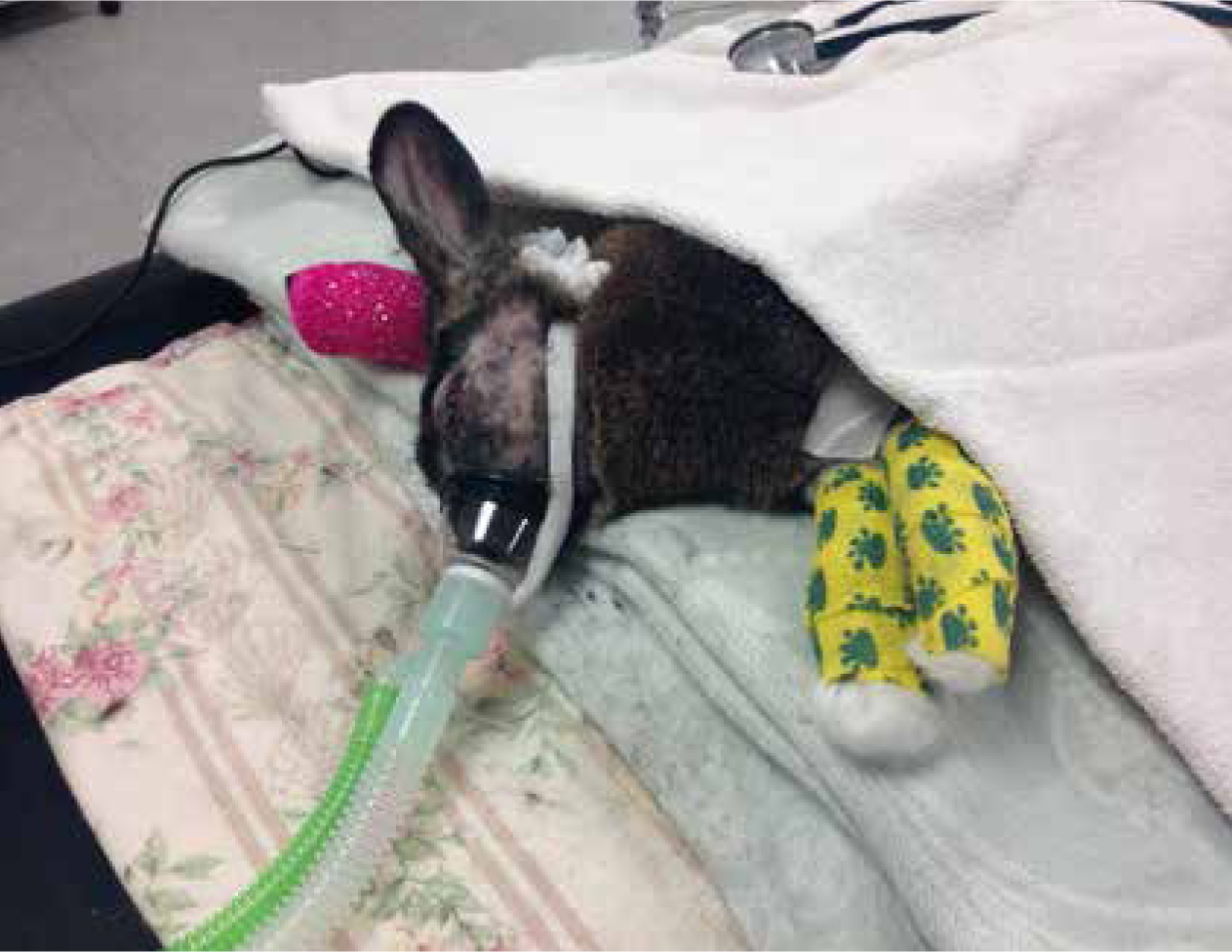
The best way to maintain normothermia in these patients, however, is to reduce the heat lost rather than trying to rewarm the patient; this can be achieved by wrapping the patient's feet in bubble wrap and using blankets and heat pads as soon as the patient is sedated (Figures 4 and 5). Care should always be taken to ensure the patient is not placed directly onto a heat source due to risk of burns. An indwelling rectal thermometer would be beneficial to monitor the patient's temperature throughout any procedures.


Stress
The link between stress and poor outcomes in hospitalised rabbits is commonly reported. In a study by Harcourt-Brown and Harcourt-Brown (2012), a link between rabbits exhibiting signs of stress and increased blood glucose levels was identified: 58% of the 907 rabbits studied showed passive or active signs of stress, such as remaining immobile, unresponsive, struggling or trembling and presented with blood glucose levels significantly higher than those that did not exhibit any stress signs. It has been suggested that hyperglycaemia is a result of the release of glucocorticoids in response to stress (Mancinelli, 2015a), this increases the blood glucose level by stimulating gluconeogenesis and promoting insulin resistance. In another study, a link between the occurrence of hyperglycaemia and hyponatraemia in rabbits was identified (Bonvehi et al, 2014). This study concluded that more research was necessary to determine whether natraemia variations elicited changes in glycaemia or vice versa. Regardless of which occurs primarily, the study determined that patients with hyponatraemia were at a significantly higher risk of mortality than normonatraemic patients. This was due to the resulting cerebral oedema leading to symptoms such as depression, convulsions, coma and death. The results of these two studies demonstrated the potential physiological effects of stress on the rabbit.
Mancinelli (2015a) stated that rabbits are considered catecholamine-driven animals, making them very susceptible to stress. Catecholamines result in the stimulation of the sympathetic nervous system causing tachycardia, hypertension and reduced renal perfusion. Mancinelli (2015b) determined that the stimulation of the sympathetic nervous system in an already unhealthy patient can result in cardiac failure. Sympathetic nervous system stimulation can also result in the slowing of the gut motility (Harcourt-Brown, 2010), this results in the gastrointestinal contents drying and becoming impacted, the contents then ferment resulting in the production of gas pockets which can cause painful gastric dilation.
A number of people have stressed the importance of ensuring that hospitalised rabbits are not separated from their companions to reduce their stress during the period of illness (King, 2008; Meredith, 2009; Moyes, 2014; Rosewell, 2015). Wild rabbits spend 40–50% of their time in contact with their companions, therefore separation from a companion is likely to cause a large degree of stress (Mancinelli and Bament, 2014). In a study into group stability in breeding rabbits, reintegration of rabbits into a group after 12 days of isolation resulted in the increased acquirement of lesions and antagonistic actions in the group (Andrist et al, 2012). This indicated that allowing a bonded pair of rabbits to be hospitalised together would reduce the chance of aggression when the rabbits are returned to their normal environment. In cases where it is not possible to have a bonded pair hospitalised together, due to wounds or illness, both rabbits may still be admitted and placed in adjoining or facing kennels and be allowed supervised playtime where appropriate. Care should be taken to ensure that this is safe for each rabbit to avoid the spread of disease in infectious cases.
It is understood that rabbits are prey species (King, 2008; Meredith 2009; Harcourt-Brown, 2010; Hedley 2011; Rosewell, 2015) and as a result of this the accommodation they are placed in while hospitalised can have a direct effect on their health and recovery. As previously discussed, the effects of stress can have detrimental physiological effects on the patient and therefore reducing it through simple methods such as correct accommodation can have a large impact on the patient's wellbeing. It has been recommended that rabbits should be housed in a separate ward away from the sight and sound of other predator species, the kennel should be at floor level and the rabbit should be provided with a bolt-hole, such as a cardboard box or the patient's carrier (Hedley, 2011; Rosewell, 2015). While separate accommodation would be ideal, not all practices have access to a designated rabbit ward, in this case the practice could accommodate rabbits in a covered, collapsible cage in a quiet area of the practice with minimal through traffic to avoid having to place them in close proximity to predator species (King, 2008) (Figure 6).

It is commonly reported that rabbits do not tolerate handling well, and as they are prey species rabbits become stressed when held above ground level (Meredith, 2009; Rosewell, 2015; The Rabbit Welfare Fund, 2016), and any interventions performed on hospitalised rabbits should be minimised. Any interventions should be grouped to avoid frequently disturbing the patient and observations should be made from a distance; the behaviour of rabbits is altered in the presence of people (Rosewell, 2015). When it is necessary to examine the patient the examination should take place on the floor with a non-slip surface to prevent injury. It is important for the veterinary nurse to be aware that the behaviour of rabbits will be altered in their presence, to reduce the risk of behaviours suggesting pain or stress being overlooked, observing the patient from a distance initially allows better observation.
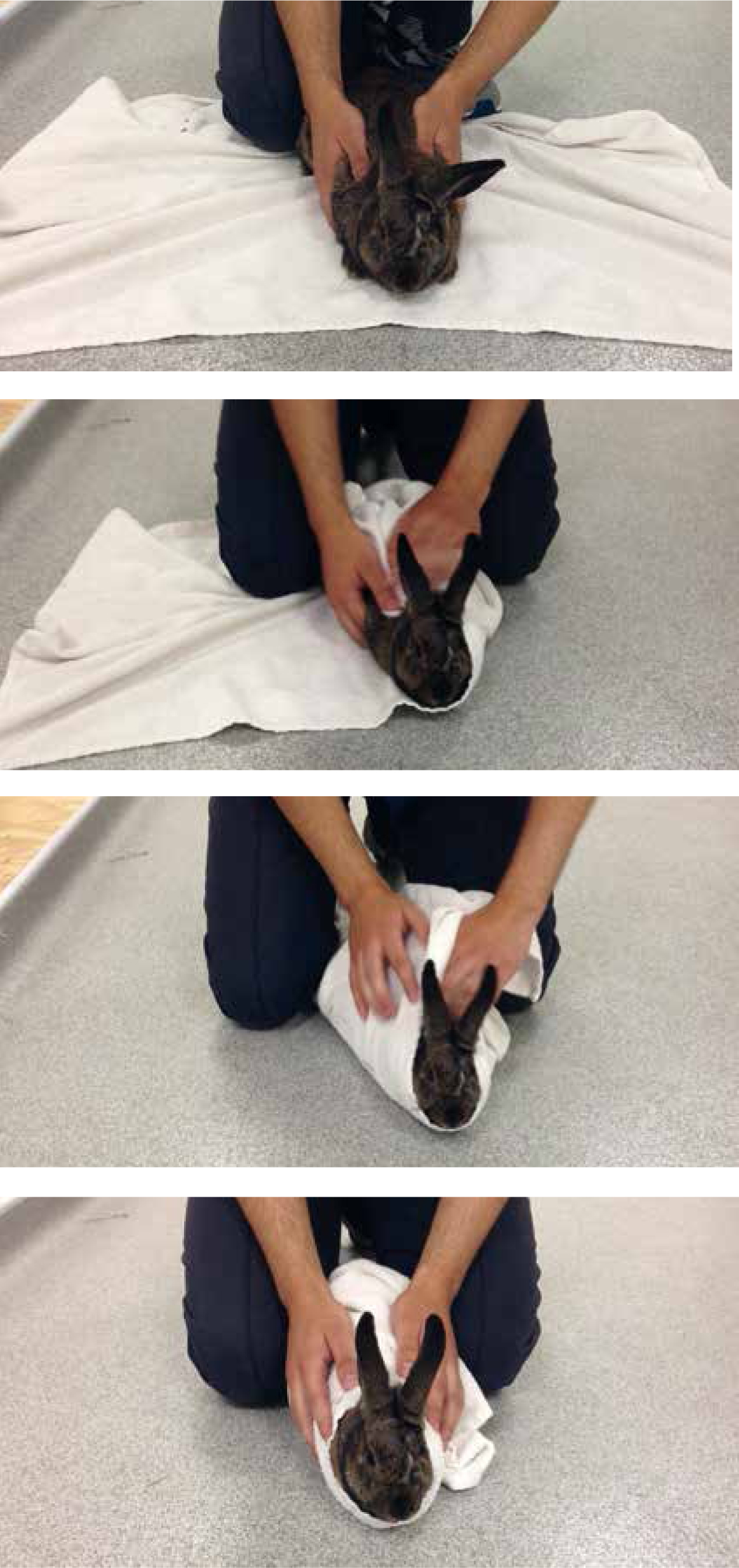
The manner in which the patient is held can also increase distress, the patient should be held close to the body with both the hind limb and ventrum supported (Rosewell, 2015). This is suggested due to the fragile nature of the rabbit's skeletal system which is prone to fractures; the rabbit's skeleton makes up only 7–8% of its bodyweight (Garner-Richardson et al, 2014). The use of ‘tonic immobility’ for the restraint of rabbits for non-painful procedures has been suggested (Harcourt-Brown, 2002), however others advised against this method of restraint as it is a fear response and causes stress (Sayers, 2010). A 2005 study by McBride et al described an increase in plasma corticosterone levels in rabbits induced into tonic immobility, suggesting the procedure caused the patient stress. This information suggests that rabbits should not be restrained in this manner, as it can result in the patient becoming stressed, more appropriate methods such as restraint using a towel are safer for the patient and are equally effective (Figure 7).
Anorexia
It is essential for veterinary nurses to understand the consequences of anorexia, to ensure appropriate monitoring is carried out to identify any issues early and allow rapid treatment to prevent patient deterioration. Anorexia in rabbits carried a very poor prognosis if inadequate treatment was provided, due to the physiological effects of starvation on the rabbit (Murray, 2006). Risk of endotoxic shock has been reported as a result of bacterial proliferation and bacterial translocation in anorexic rabbits (Steinmetz and Clauss, 2010; Rosen, 2011). A frequently described consequence of anorexia is the incidence of hepatic lipidosis (Murray, 2006; Redrobe, 2007; Steinmetz and Clauss, 2010; Rosen, 2011; and Harcourt-Brown, 2011). After a prolonged period of anorexia, free fatty acids become mobilised from adipose tissues to be used as an energy source, the patient enters a negative energy balance, the free fatty acids are sent to the liver to be metabolised and accumulate in the hepatocytes resulting in hepatic lipidosis, and eventually liver failure (Harcourt-Brown, 2010). There is also increased risk of ketoacidosis in rabbits over other species due to the differences in their metabolic pathways; as they are unable to react efficiently to the metabolic acidosis it can easily result in death (Harcourt-Brown, 2010). It is suggested that the occurrence of ketoacidosis is a result of the oxidation of the free fatty acids secondary to hepatic lipidosis, therefore the prevention of hepatic lipidosis is likely to help with the prevention of ketoacidosis (Harcourt-Brown, 2005b). The administration of early enteral nutrition is supported to prevent hepatic lipidosis and the increased potential for mortality. The veterinary nurse plays a vital role in the treatment of anorexia through regular syringe or naso-oesophageal meals, through tempting the patient to eat with palatable foods, and providing an environment conducive to recovery. When syringe feeding patients, a palatable recovery food should be used, the patient should be wrapped in a towel and given approximately 1 ml at a time, then allowed time to swallow. Feeding large amounts of food rapidly can distress the patient and could result in aspiration pneumonia if any of the food is inhaled (Hernandez-Divers, 2005). Close monitoring of food and water intake is essential and it may be necessary to weigh food in order to accurately record the patient's intake. As well as this, monitoring faecal output is also important; the size, shape and consistency of any faeces produced should be recorded and the patient should be checked regularly for gut sounds.
Pain
It is generally agreed that the provision of pain relief is essential to prevent the suffering and further deterioration of hospitalised patients (Harcourt-Brown, 2005a; Lawton, 2005; Meredith, 2009; Mancinelli, 2015b). The mechanisms for nociception in animals closely resemble that of humans, therefore it can be assumed that any procedures or conditions that would be painful to a human, would also be painful to a rabbit (Lawton, 2005). As animals are unable to perceive an end to pain, the experience may actually be worse for them than for humans (Reid et al, 2013), therefore the need for effective analgesia should not be underestimated. One of several detrimental effects of untreated pain in rabbits is a lower immune response, which ultimately increases the rate of mortality in this species (Barter, 2011). It is essential for veterinary nurses to understand the consequences of unmanaged pain and to ensure that it is identified and analgesia administered swiftly to avoid the harmful effects. Identification of pain can be difficult in prey species; however the use of the grimace scale could assist veterinary nurses in determining if patients are suffering by assessing their expression and body language (Keating et al, 2012).
Ensuring that the patient is provided with suitable analgesia is essential and consideration must be taken to the type of analgesia provided. Some authors advocate the use of opioid analgesia (Lawton, 2005; Harcourt-Brown, 2005a), while others report that rabbits are particularly sensitive to the respiratory and gastrointestinal complications associated with their administration (Mancinelli, 2015b). Several analgesics have been suggested for use in rabbits including, butorphanol, buprenorphine, pethidine and meloxicam (Fraser and Girling, 2009). Veterinary professionals should be aware that many analgesics are not licensed in rabbits and therefore the cascade must be followed and the owner must consent to the use of an unlicensed medication before they can be administered (NOAH Compendium, 2015). The use of pre-emptive and multi-modal analgesia are essential in order to manage pain effectively. A suggested protocol includes the use of an opioid, a non-steroidal anti-inflammatory drug (NSAID) and a local anaesthetic in combination to manage pain in rabbits undergoing surgery (Mancinelli, 2015b) (Figures 8 and 9).
Recommendations for future studies
While there are many suggestions regarding the solutions for the reduction of rabbit fatalities, many of these are based on the veterinary professional's experience and studies into other species as opposed to rabbits. Many veterinary practices do not have access to a designated rabbit ward (King, 2008). Further research is necessary to determine whether veterinary practices in this position sustain a higher percentage of fatalities. Despite the fact that extensive research has determined that early enteral nutrition is instrumental in the recovery of patients suffering from anorexia or gastrointestinal disease, many experts suggest different methods and timing of food administration as superior for patient recovery. Further research is required to determine the best method of food administration for patients in the initial stages of anorexia or gastrointestinal disease.
Recommendations for practice
The provision of clear practice protocols should be in place to ensure an optimum level of care is provided to all patients. Protocols should indicate the correct handling of rabbits to reduce the instance of spinal fractures and stress as a result of inappropriate handling. Where possible the patient should be restrained for interventions on the floor, on a non-slip surface to reduce the chance of injury through falls (Rosewell, 2015). Regular continued professional development should be encouraged by practices, to allow the implementation of the most up-to-date evidence-based nursing care. This will also increase the number of veterinary nurses comfortable with the care of rabbits which will be beneficial to the rates of survival. As the postoperative period was identified as the highest risk for fatalities by Brodbelt et al (2008), veterinary nurses should be encouraged to follow their patient through to recovery where possible, this would allow the early identification of any deterioration of the patient. The use of multimodal, pre-emptive analgesia for rabbits undergoing surgery is encouraged, a combination of an opioid and NSAID should be administered with the addition of local anaesthetic where necessary, and a Grimace scale should be employed to identify pain (Keating et al, 2012).
Conclusion
There are many factors that can affect the survival of hospitalised rabbits in practice, however the main findings can be summarised into five categories including anaesthesia, temperature, stress, anorexia and pain. From the information ascertained it is evident that none of these factors are singularly more relevant to patient mortality than another. Instead each factor has a direct effect on the others and while individual factors can result in fatalities, the results from a combination of factors will produce a much higher incidence of death. It is therefore clear that the care of rabbits in the veterinary practice must be holistic and consideration must be given to every aspect of care in order to decrease the rate of fatalities in this species. It would appear that many of the fatalities of rabbits could be prevented with the utilisation of proper protocols and staff training. The information obtained in this review indicated that, with the provision of extensive care from knowledgeable and committed veterinary professionals, the rate of fatality could be decreased. As this species of pet continues to become more popular, the veterinary profession will be under more pressure from owners to provide care to a standard equal to that provided for canines and felines. Further research and continued training for staff will hopefully provide better care in the future.

