References
Fibroadenomas in rats
Abstract
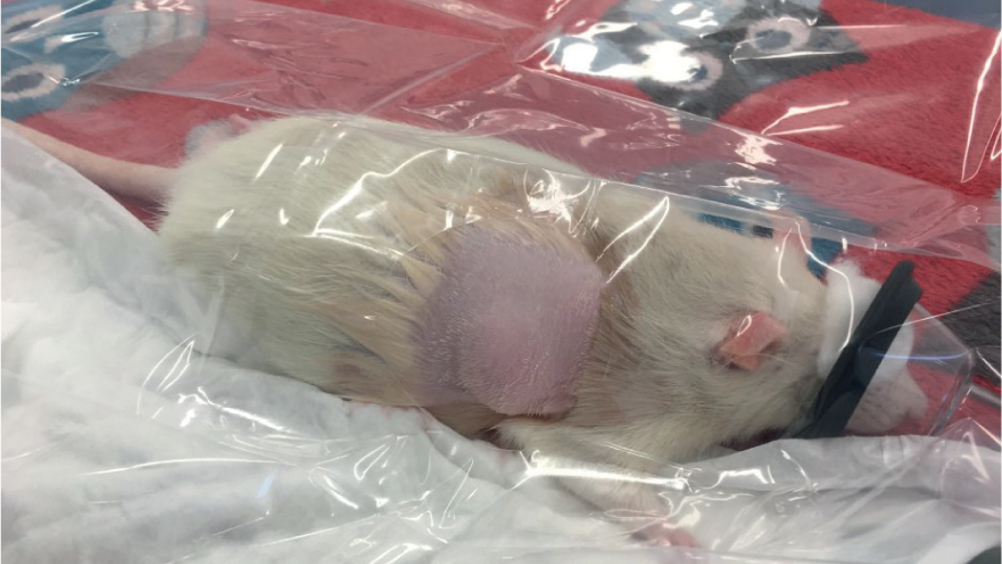
This article looks at one of the most common reasons for presentation to practice in rats, mammary fibroadenomas. There is an extremely high incidence of this disease process in rats over 1 year of age and veterinary nurses have a duty of care towards these patients to provide up-to-date nursing techniques and engage in clinical discussions with veterinary surgeons to implement appropriate treatment plans. Surgical and medical options are explored along with tips for successful anaesthetic management of these patients. Although these neoplasms are benign, they are often fast growing and can significantly affect quality of life in rat companions.
Mammary masses are one of the most common reasons for presentation to practice in female rats. A high incidence (80–95%) of these masses are benign fibroadenomas (Percy and Barthold, 1993) with this diagnosis coinciding with a high incidence of concurrent pituitary tumours. More rarely, adenocarcinomas and interstitial cell tumours can present, which carry a poorer prognosis. Because of the high incidence of fibroadenomas, these will be the focus of this article along with their management and prognosis. Male rats can also be affected, but this is less common as mammary growths are primarily stimulated by prolactin secretion and indirectly affected by oestrogen.
Fibroadenomas are known to be under hormonal influence, more specifically it is theorised that high levels of prolactin from prolactin secreting pituitary adenomas influence the development of these neoplasms (Bennett, 2012). A study by Hotchkiss (1995) concluded that oestrogen does not appear to increase the incidence of tumour development itself, but may be a contributing factor in the development of pituitary tumours, which in turn increases the incidence of mammary tumours. Ovariectomised rats (at 90 days of age) had a significantly lower incidence of pituitary adenomas than intact females (Hotchkiss, 1995); Hoefer and Latney (2011) reported a 90% incidence of fibroadenomas with concurrent pituitary tumours. In a study by Planas-Silva et al (2008) ovariectomy performed between 5–7 months reduced the incidence of spontaneous mammary neoplasms by 95%. More positively ovariectomy also increased the 110-week survival rate and overall lifespan, which could potentially be a result of a lesser incidence of pituitary tumours, however this was not analysed. Currently ovariectomy in general practice is not routinely carried out in female rats. This is likely for a variety of reasons. Owners rarely present rats to practice unless there is a perceived problem and, in the author's experience, new pet checks and discussion of preventative medicine is rare in rodent species. Financial implications for the client and the perception and reality of the risk of small mammal anaesthesia and surgery create barriers to performing such procedures. It may often be concluded that removal of a demarcated mass later in life is a simpler procedure than ovariectomy, however the majority of the former patients are geriatric and, in reality, likely pose a higher anaesthetic risk. Further evidence-based studies involving pet rats are likely required to confidently encourage prophylactic ovariectomy in this species.
Register now to continue reading
Thank you for visiting The Veterinary Nurse and reading some of our peer-reviewed content for veterinary professionals. To continue reading this article, please register today.

