Reptiles are very different to the cats and dogs usually seen in practice, but with some consideration of the anatomy and physiology of the various species, these patients can be anaesthetised with minimal risk. This article will cover the basics of how to safely anaesthetise, monitor and recover the pet reptile species most commonly seen in veterinary practice.
The importance of heat
One of the most important things to remember when it comes to reptiles is that they are ectothermic, meaning that they require an external heat source (i.e. the sun) to be able to perform normal metabolic bodily functions such as digestion. It is extremely important that these species remain within their preferred optimum temperature zone 24 hours a day (Table 1) to maintain health. Being maintained at the correct temperature will mean that they are able to metabolise drugs and fluids and this will ensure a much smoother and predictable anaesthesia. When housed in the hospital environment, reptiles should be maintained at their optimum temperature either by using supplementary heating within a vivarium set up or a modified kennel with a heat lamp (Figure 1). Both heat lamps and heat mats can be used to raise the background temperature of the environment, heat sources should be connected to a thermostat to prevent overheating and a thermometer should be used to monitor the temperature.
| Species | Preferred optimum temperature (°C) |
|---|---|
| Spur-thighed tortoise, Hermann's tortoise | 20–28 |
| Red-eared terrapin | 20–24 |
| Bearded dragon | 25–35 (up to 42 in basking spot) |
| Green iguana | 25–35 |
| Leopard gecko | 25–30 |
| Veiled chameleon | 21–38 |
| Corn snake | 25–30 |
| Boa | 28–30 |
| Royal python | 25–30 |

Premedication
A premedication may be advisable depending on the procedure; often this is in the form of an analgesic such as a non-steroidal anti-inflammatory drug or an opioid if painful surgical procedures are to be performed. Analgesia use in reptiles is difficult due to the lack of research into the many species making up this group, therefore the types of drugs available to use are limited. Pre-anaesthesia sedatives can be used prior to induction depending on the veterinarian's preference, but it should be noted that drugs such as ketamine have been associated with prolonged recovery (Schumacher and Yelen, 2006).
Induction
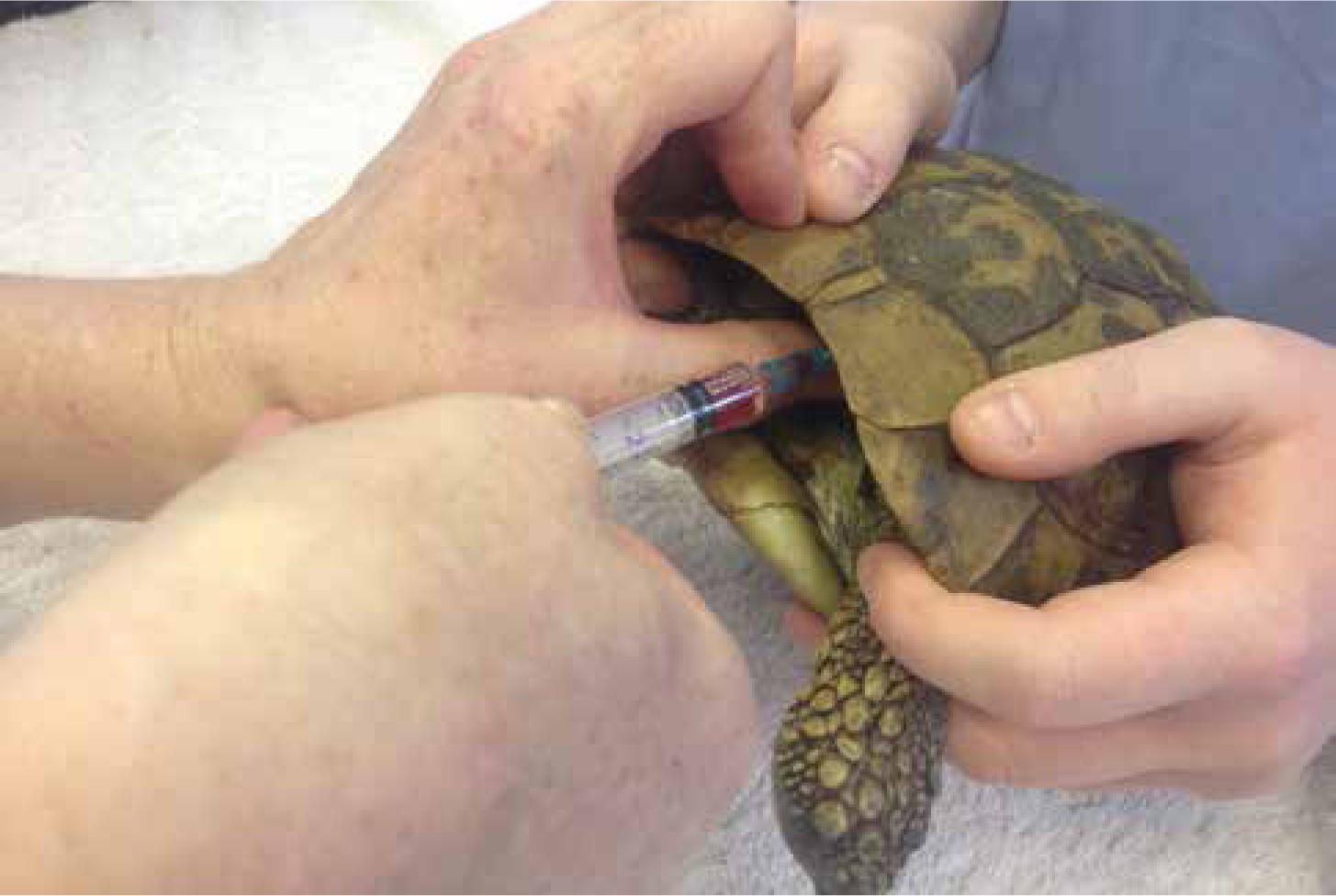
Induction in reptiles can be achieved by the intravenous route. Both propofol and alfaxalone are used in reptile species as an induction agent. The dosages vary slightly (Table 2) for different species. The main points for venous access on species are:
| Drug | Dosage | |
|---|---|---|
| Alfaxalone | 2–4 mg/kg | IV Higher dosages required for intramuscular administration |
| Butorphanol | 0.5–2 mg/kg IM | |
| Dexmedetomadine | 100 µg/kg SC | In combination with other sedatives |
| Isoflurane | 3–5% for induction, 2–4% maintenance | |
| Ketamine | 2–10 mg/kg IM/IV | Use in combination with other sedatives, not used alone ideally as long recoveries reported |
| Medetomadine | 100–200 µg/kg IM | Can be used with or without other drugs |
| Midazolam | 1–2 mg/kg IM | Usually in combination with other sedatives |
| Morphine | 1–4 mg/kg IM | Likely to cause respiratory depression |
| Propofol | 5–10 mg/kg IV | |
| Sevoflurane | 6–8% induction, 3–5% maintenance |
IV, intravenous; IM, intramuscular; SC, sub cutaneous
Other methods of induction of reptiles include an intramuscular injection of alfaxalone or drug combinations such as medetomidine and ketamine and then intubation of the patient once sufficiently sedated. Combination induction agents may prove to be more predictable than the use of single induction agents due to blood shunting in the heart which can bypass gaseous anaesthesia (discussed later in this article). Gaseous induction in a chamber can be successful in smaller snakes and lizards, but this can take an extended period of time as reptiles have conscious control over their ventilation and also have the ability to survive long periods of time without oxygen (O2), turtles being the most apt at this (Bickler and Buck, 2007). Recent discussions suggest that often multi-modal anaesthesia is better in reptile species (Nevarez, 2016).
Intubation
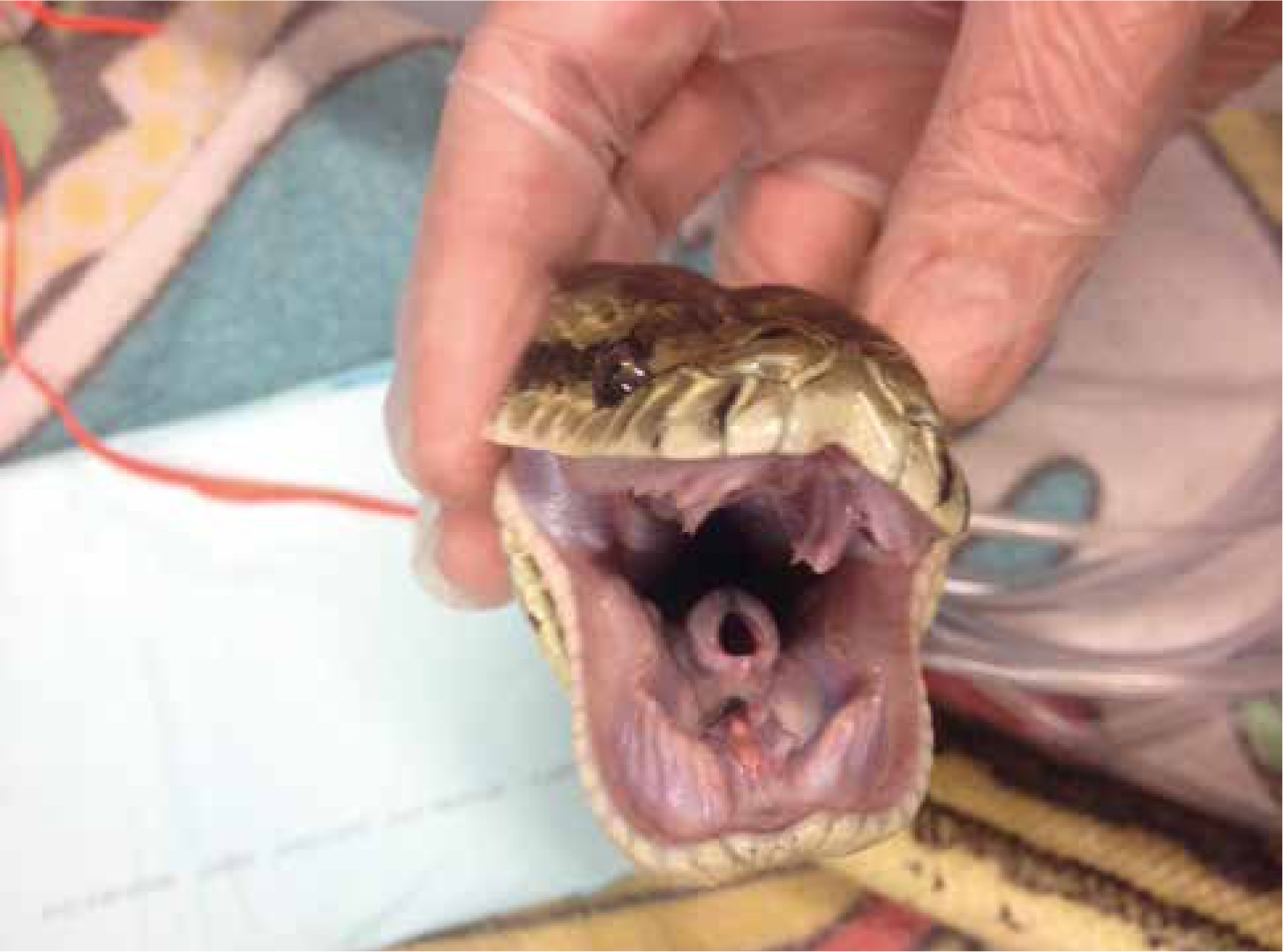
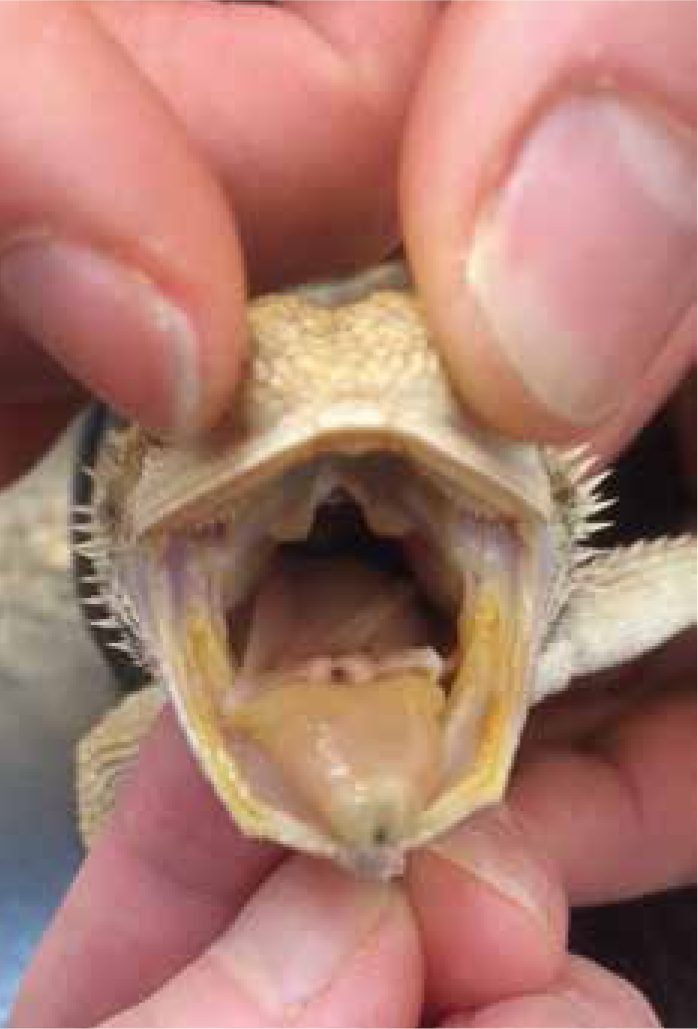
It is vital that any reptile undergoing full anaesthesia for a long procedure is intubated. As reptiles have conscious control over their ventilation once they become anaesthetised, their ability to breathe becomes suppressed and often ceases. The glottis of most pet reptiles is relatively easy to visualise because they do not have an epiglottis, snakes for example have an easily visualised opening to their trachea (Figure 4), and many reptiles have a fleshy easily visible glottis at the base of the tongue (Figure 5). Using a stylet introducer (such as a dog urinary catheter) can be advantageous in smaller species as the larynx will remain closed at rest until the patient ventilates and it will be difficult to pass a endotracheal tube into. Local anaesthetic sprays are not usually required as there is no evidence to suggest reptiles suffer from laryngospasm.

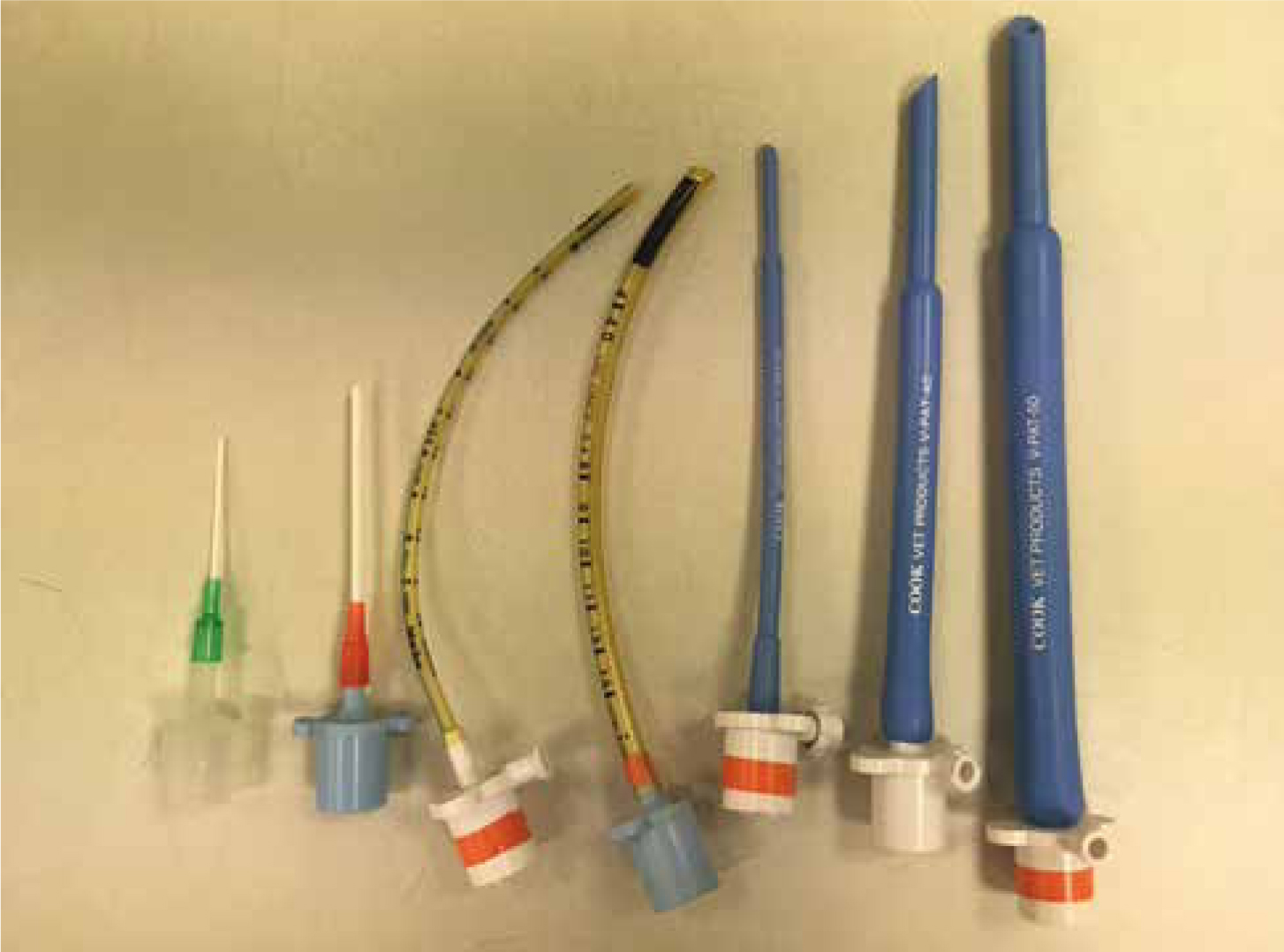
Often the endotracheal tube (ET) of choice in reptiles are uncuffed due to the concern of causing damage with cuffs. Cook tubes designed for exotic species are advantageous, these tubes have a narrower end and can be useful at creating a seal over the larynx. In cases of very small species, adapting an intravenous catheter to make a small tube is acceptable (Figure 6). The tube should sit snuggly into the trachea without causing excess damage. It is also important to be aware of any species‘ specific anatomical differences. For example, in chelonians the trachea bifurcates very cranially and it may be possible to accidently intubate one bronchus, for this reason it is advisable not to push the tube too far down. Tubes can be tied in place with regular bandage, but often a combination of tape and the use of a tongue depressor as a stabiliser can be a useful way of securing the tube in place and can also help prevent snagging on any teeth.
Care should always be taken with intubating reptiles that are only sedated, as many species have teeth and/or powerful beaks which can cause trauma to fingers.
Ventilation
The reptile's stimulus to breathe is low levels of O2; when attached to an anaesthetic circuit with 100% O2 flow these patients lose the ability to spontaneously ventilate. Once the patient is intubated it needs to be maintained on mechanical or manual intermittent positive pressure ventilation (IPPV). Mechanical ventilation, which is less labour intensive and more controlled, can be achieved by placing the patient onto a small animal ventilator (such as the Vetronics SAV04 in Figure 7). Reptiles tend to have a higher tidal volume than mammals, and the rate for ventilation in most reptiles should be around 4–8 breaths per minute, which can be increased to help deepen the anaesthesia. However due to some degree of cardiac shunting, sometimes increasing the rate has no effect on anaesthesia depth. Pressures for ventilation should not exceed 10–15 cm H2O. (Schumacher and Yelen, 2006). Care should be taken to watch the patient's body wall and mimic the natural movement of breathing. Chelonian species will maintain ventilation by movements of the forelimbs so this should be mimicked during IPPV.
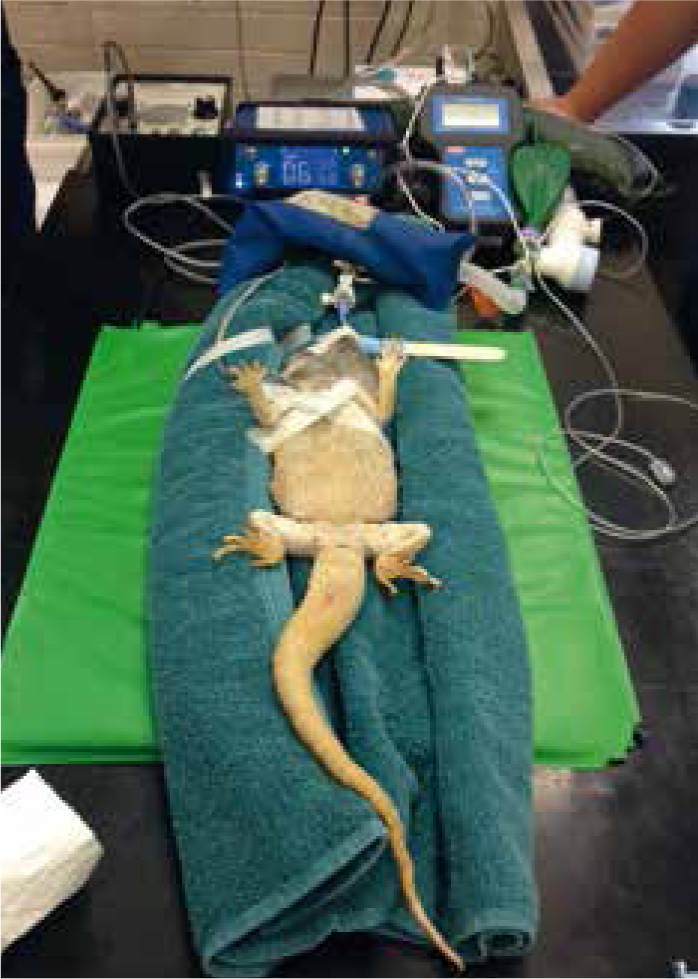
Monitoring
Depth of anaesthesia can be difficult to assess in reptiles as reflexes are often unreliable. Reptiles have a three-chambered heart which means that both oxygenated (with anaesthetic agent) and the deoxygenated blood mix within the ventricle; they have some control over this and therefore gaseous anaesthetic agent can be bypassed out of the body and not absorbed at all. Most reptile lungs are very primitive as many do not possess large numbers of alveoli and are often more like simple air sacs than the advanced lungs seen in mammals. This means less perfusion of gas; because of this and heart shunting reptiles tend to require a higher percentage of volatile agent (Sladky and Mans, 2012). This is especially so if they have not had a prior sedative premedication, and anaesthetic vaporizer will need to be turned onto the highest percentage of gaseous anaesthetic for several minutes prior to any surgery beginning.
A Doppler probe should be placed onto the patient's apex beat so that the heart rate can be monitored throughout anaesthesia, this is useful so that all assisting with the procedure can audibly monitor the heart rate. Many lizard species' hearts are very cranial; for chelonian species a Doppler trace can be obtained by placing the probe onto the carotid artery area (Figure 8). Stethoscopes are not useful at heart rate monitoring as they will not usually be able to pick up acoustics through reptiles' thick scaled skin.
Capnography can be used and often readings will be low. This is due to reptiles being so good at dealing with long hypoxic episodes and the fact that they need less O2 to perform bodily functions. Due to blood shunting the expired carbon dioxide (CO2) levels are not necessarily equivalent to arterial pCO2 levels and attention can be paid to trends rather than numbers. Pulse oximetry is difficult to obtain due to the scaled skin, oesophageal probes are available in this case. The temperature of the environment should be monitored and ideally kept at the patient's preferred optimum temperature, a rectal probe can be used to obtain the patient's core body temperature
See Table 3 for tips on monitoring reflexes during anaesthesia.
| Light/sedation anaesthetic plane | Moderate anaesthetic plane | Deep anaesthetic plane |
|---|---|---|
| Righting reflex sluggish, withdrawal reflex sluggish, cloacal reflex present, jaw tone present, spontaneous ventilation (often reduced), tail pinch absent in snakes but head end awake | No righting reflex, no withdrawal reflex, cloacal reflex sluggish/absent, jaw tone absent, apnoeic, heart rate may change or remain the same, may make limb movements to painful stimuli during surgery | No righting reflex, jaw tone absent, no limb movement, no cloacal reflex, heart rate slower, apnoeic, corneal reflex can be present until death |
Recovery
Reptiles, because of their slow metabolism, tend to take much longer to recover than mammals. A recent study in ball pythons (Python regius), for example, showed the longest time to recover from a 60 minute gaseous anaesthesia was close to 6 hours (Jakobsen et al, 2017). Staff and owners should be made aware of this prior to anaesthesia, as the patient will need to remain in hospital to recover. Patients should remain intubated and ventilated in recovery. Due to their lower O2 demand, it is advisable to manually ventilate patients in recovery on room air or CO2 to stimulate ventilation with lower levels of O2 (as mentioned previously, the respiratory stimulus in reptiles is low partial pressure of O2). This is often achieved by using an Ambu bag for manual ventilation (Figure 9). Reflexes should be monitored throughout.
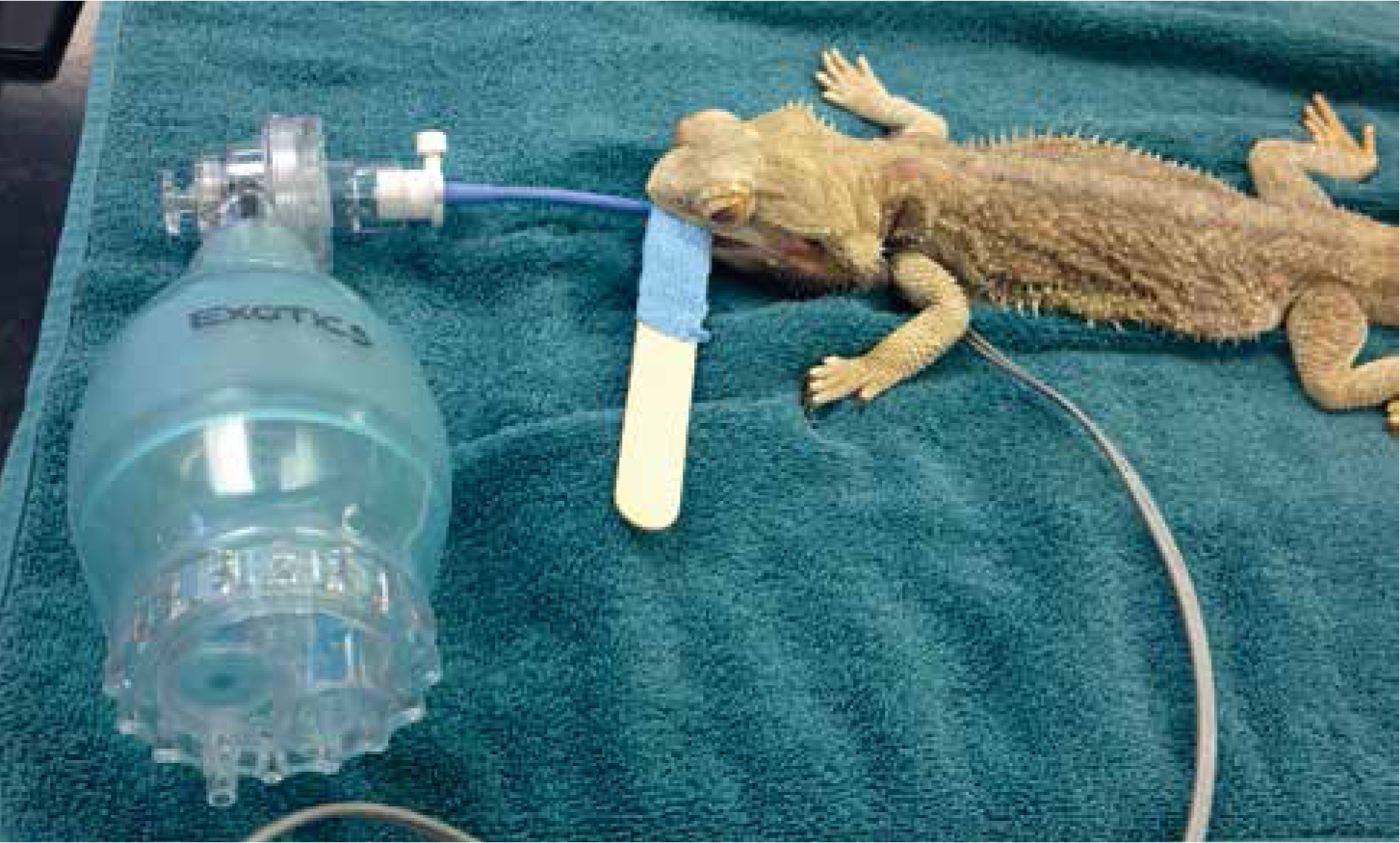
Patients can be actively warmed using a hair dryer. This is useful not only for warming the patient, but also for rousing them from unconsciousness, and helps to aid a quicker recovery. The patient should remain intubated until they are adequately ventilating for themselves. At this point they can be extubated and placed back into their enclosure. If the patient is not very mobile they should not be placed directly onto or under a heat source as they are likely to overheat, this can also increase their O2 demands which may not be met by their reduced rates of ventilation. Environmental temperatures should be monitored throughout recovery.
Conclusion
Reptiles have completely different body systems to that of mammals and it is important to take this into consideration when anaesthetising this group of animals. If unfamiliar then staff should research the species in question. These patients should always be kept at their optimum temperature to administer medications and should always be ventilated during long procedures despite their hypoxia tolerances. Taking these factors into considerations, successful anaesthesia can be achieved.
