Inflammation is a crucial and normal part of the healing process and is characterized by redness, production of exudate, swelling and pain (Table 1). Inflammation delivers neutrophils and macrophages to the wound site and enables clearing and natural debridement of debris, bacteria, and devitalized tissue (Shultz, 2007).
| Haemostasis (stemming of bleeding) | Bleeding stops and as clotting progress platelets begin to adhere to the site of injury releasing chemical messengers to begin inflammation |
| Inflammation (natural debridement) | Neutrophils and macrophages begin clean up process. Slough and exudate produced as a by product. Inflammation most active during 2-3 days after injury. As the process dies down it triggers proliferation |
| Proliferation (true healing) | Vascular tissue begins to proliferate at the base of the wound to provide a healthy bed for epithelialization. Fibroblasts stimulate wound contraction and are essential for rapid closure. Surgery and reconstruction may be used to bypass this process by bringing wound edges together |
| Maturation (scar maturity) | Collagen laid down during granulation and used as a structural matrix by epithelial cells is replaced with a more organized collagen. This provides the wound with greater tensile strength. This process will continue many months post injury and the wound may only be 80% its original strength after 1 year |
Powerful enzymes called proteases are used during this process to break down large proteins into a more liquid form for easy digestion by macrophages. As a result the wound takes on a more goey, sloughy appearance as inflammation peaks at around 2 to 3 days after injury (Collier, 2003; Shultz, 2007).
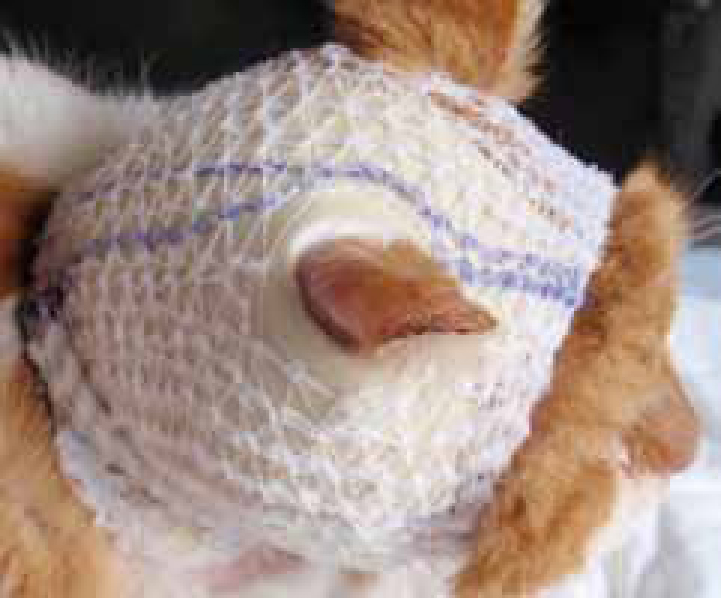
Production of slough and exudate during the inflammatory phase is the reason a fresh wound will often look ‘worse’ before it starts to look better. However, it is possible to confuse these symptoms with infection (Figure 1).
Infection is closely associated with inflammation and is brought about by bacteria, fungi, yeasts or virus that proliferate in the wound environment (Collier, 2004). In most wound infections bacteria are the likely cause (Remedios, 1999).
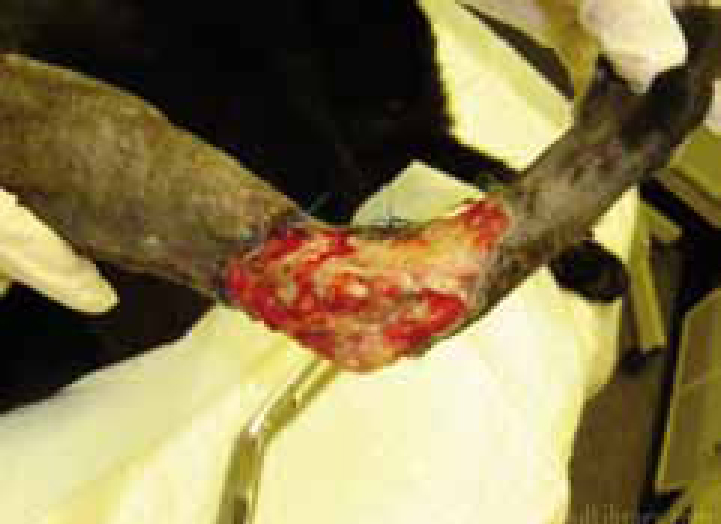
Organic and contaminated material, such as soil, grit and hair are likely to be introduced to wounds during trauma. Responsible for prolonging the inflammatory response the macrophages struggle to digest and phagocytose the debris. A foreign body effect may further create a pocket of inflammation that persists and an absc Mess or sinus may result (Figure 2).
The presence of a few bacteria in a wound does not mean there is an infection (Table 2). Bacteria are introduced into every wound environment, even when the best aseptic technique is used. It is not until they start to thrive and initiate a host reaction that the wound is said to be infected and symptoms develop.
| Bacterial load | Clinical significance | Symptoms in wounds |
|---|---|---|
| Contamination | None — normal scenario. Local bacteria accidentally present in the wound, but not yet multiplying to a point that affects healing | None — normal status of presenting wounds. Avoided in surgical wounds through fastidious aseptic technique |
| Colonization | None — normal scenario in slightly older wounds open wounds open to the air. Bacteria have colonized the wound, but are not in quantities high enough to affect healing | No symptoms of infection. Inflammatory phase of healing may be of extended duration |
| Critical colonization | A hypothetical mid point where bacterial load and immune response is in stasis. Healing is likely to be halted, but there will be no overt signs of infection | Traumatic wounds may fail to respond to dressings and appear to be ‘stuck'. Surgical wounds may fail to unite. No clinical signs of infection will be present |
| Local infection | Inflammatory changes respond to a burden of bacteria that are beginning to thrive in the wound environment. Visually apparent changes | Inflammatory changes will be clear and prolonged. Exudate will be increased and may be purulent. Erythema, swelling and patient sensitivity due to increased pain is likely |
| Systemic infection | Bacteria within the wound spread beyond the wound boundaries to affect the whole patient. Inflammatory response becomes systemic and critical | As above, which may be combined with spreading inflammation ‘tracking' towards the heart. Patient shows signs of systemic illness, inactivity, inappetence, malaise and an elevated body temperature |
A swab revealing that a certain bacteria is present does not mean the wound will not heal. In normal wounds there will often be contaminants, but not until they reach a volume of around 105 bacteria/cm2 and above are wounds likely to exhibit signs of infection (Krizek and Robson, 1975), but given a protein-rich, debris-heavy environment bacteria that have been implanted into the wound through trauma can proliferate (Tenenhaus, 2007). They can quickly multiply and overcome the host inflammatory response.
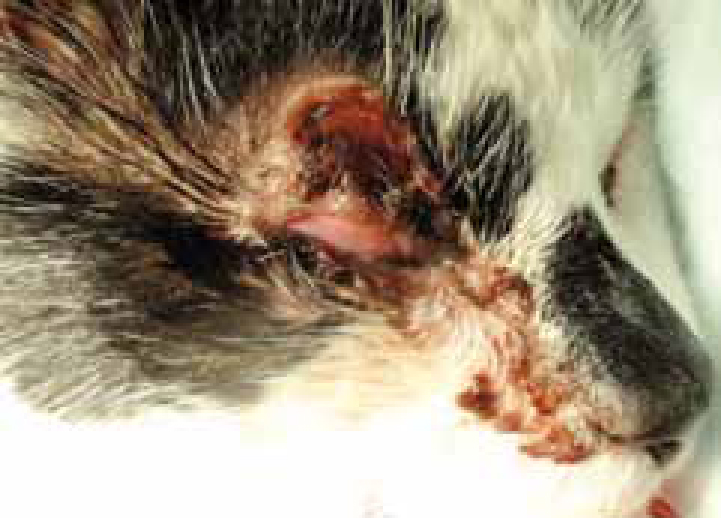
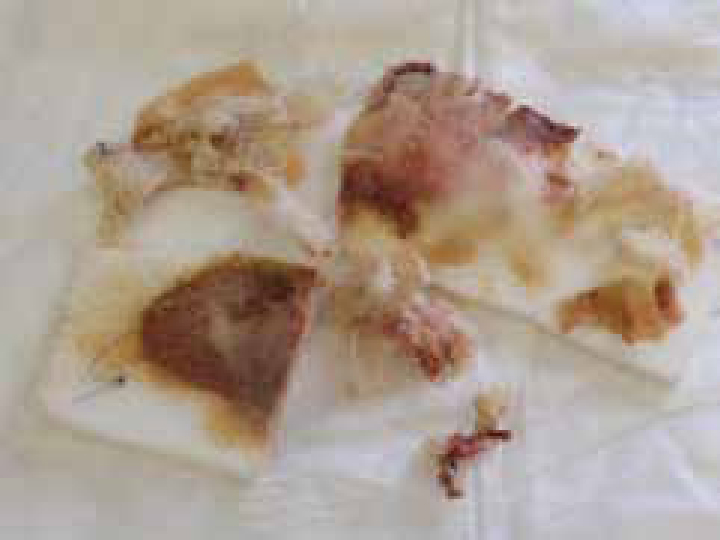
At this point it may be apparent that the wound is failing to progress to granulation, exudate may increase, it may be purulent and an offensive smell may be noticeable, a key indicator that infection is present (Figure 3; Figure 4).

Once infection becomes systemic the patient will have an elevated white blood cell count and accompanying fever. Speed of onset is dependent upon the mode of injury and the range of bacteria that are present in the wound. Puncture and bite wounds are commonly the most rapid to deteriorate due to the heavy volume of bacteria, associated tissue trauma and co-existing species of aerobes and anaerobes which help each other thrive in the anaerobic wound environment. Cat bite wounds are a typical example with Pasteurella multocida a facultative aerobe able to encourage more rapid deterioration of tissue alongside anaerobes (Hirsh, 2004). A systemic infection developing within 24 hours may not be unusual in these cases and a range of species of bacteria will likely be isolated. As a result, prophylactic antibiotics are often used for bite wounds. Culture and sensitivity may be used to confirm the antibiotic selected is appropriate. Complications can become life threatening if the effects of bacterial toxins and the subsequent inflammatory reaction overcome the patient (Dunning, 2003). Disruption of wound healing, increased patient distress, and the potential for death means that it is essential that infection is identified and managed as soon as possible.
Antibiotics, swabs and cultures
Antibiotics do not remove the burden of protein rich material and the potential contributing factors that may have encouraged infection in the first place. Bacteria present in a wound at high levels will cause the wound to fail to progress (Table 3) and swabs and cultures may be requested to confirm the species and cause of infection. They will reveal bacteria present within the wound in order for appropriate antibiotics to be prescribed, but their effect will be no substitute for removal of debris and devitalized tissue that effectively harbours bacterial colonies out of reach of any systemic treatment.
| Signs of inflammation | Signs of wound infection | |
|---|---|---|
| Redness | Redness | |
| Swelling | Swelling | |
| Increased exudate | Purulent exudate | |
| Presence of slough | Sudden increase in slough, particularly more than 4 days and onwards after injury | |
| Purulent slough — green to tan Offensive smell | ||
| Lack of progress towards healing | ||
| Pocketing and undermining of wound edges (Figure 5) | ||
| Fragile wound bleeds easily | ||
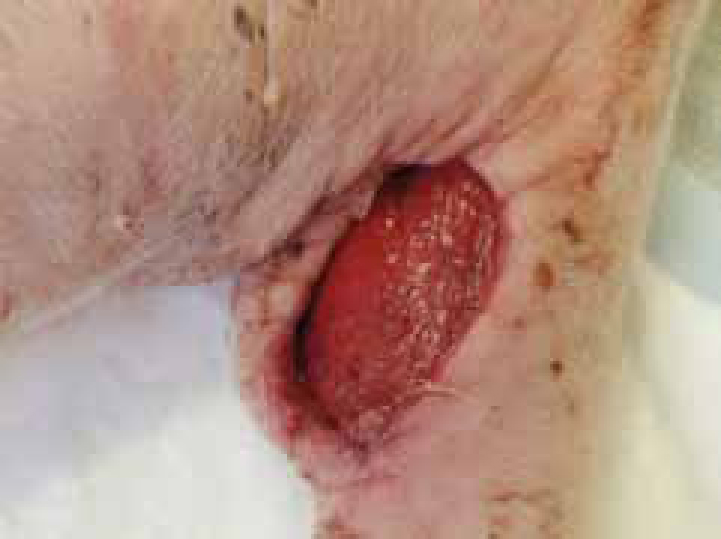
The report generated from the swabs and cultures will reveal which antibiotics the species tested are sensitive to. In the case of meticillin-resistant Staphylococcus aureus (MRSA) this may mean there are a limited range of antibiotic options (Figure 4). MRSA generates a great deal of fear among pet owners who believe their pet may not recover or will rapidly deteriorate should the wound be confirmed as contaminated, colonized or infected with the bacteria, although in the wound environment it is unlikely to be any more destructive than the sensitive version (Collier, 2004).
Pseudomonas species are, actually, more destructive to a healthy wound environment than the staphylococcal species (Percival, 2004). However, MRSA is a serious consideration, particularly for other patients who may be compromised in practice. Strict standards of hygiene and patient isolation should be adopted in order to protect other patients that may be susceptible in the clinic. For full guidance and practical guidelines in the management of patients in practice with MRSA search for MRSA on the BSAVA website at www.bsava.com, alternatively, the Bella Moss Foundation www.thebellamossfoundation.com also provides a database of papers, research and a list of advisors able to help with individual MRSA cases.
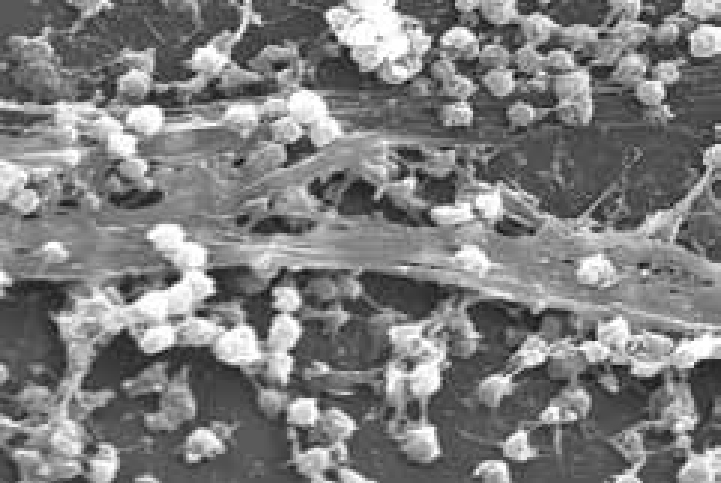
It is likely that more than one species of bacteria is present in the wound (Table 4) and in chronic wounds there may be several species forming synergistic groups or a protective biofilm layer. It is thought that the formation of biofilms in the wound can enable bacteria to resist management using standard treatments leading to persistent recurrence of infection and healing delay (Percival, 2004) (Figure 6).
| Pasteurella spp. (most specifically cats, cat bite wounds) |
| Escherichia coli |
| Clostridium |
| Staphylococcus aureus, and resistant strains |
| Staphylococcus intermedius |
| Pseudomonas |
| Streptococcus spp. |
Preventing wound infection at the earliest opportunity through effective lavage (Figure 7; Figure 8) and debridement will minimize the risk of communities of bacteria and their biofilms becoming established (Percival, 2004; Tenenhaus, 2008). The early formation of biofilms should also be prevented.

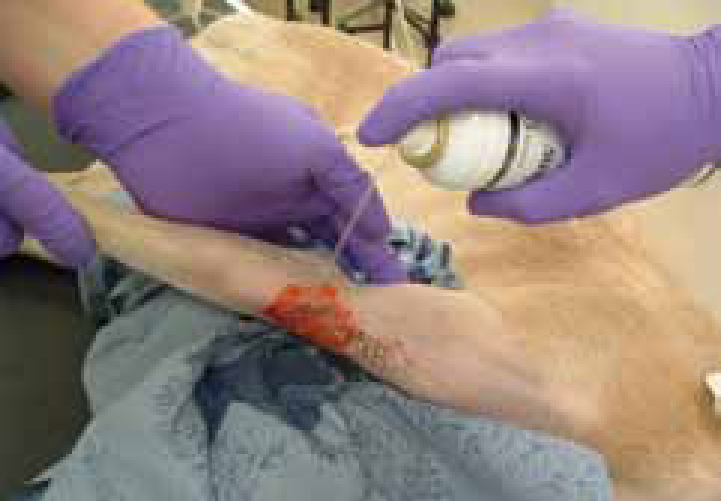
Reducing the burden: lavage and debridement
Wounds can be classified to reflect the level of contamination and the relative risk of infection if no action is taken. Removal of bacterial load and debris through lavage and debridement will effectively move these wounds from high to lower risk (Bellah and Williams, 1999; Tenenhaus, 2007) (Table 5).
| Wound | Typical wound | Infection risk | Closure options |
|---|---|---|---|
| Clean wounds | Surgical under aseptic technique | Very low | Direct surgical closure |
| Clean – contaminated | Wounds that have been thoroughly lavaged and debrided | Low | Period of open wound management to encourage granulation tissue formation. Direct closure possible if wound less than 6 hours old (bite and puncture wounds may be an exception) |
| Contaminated – dirty | Presenting wounds with clean edges and some debris, e.g. lacerations | High | Lavage, debridement and open wound management |
| Dirty | Presenting wounds containing debris, foreign material, devitalized tissue and bacteria | Very high | Lavage, debridement and open wound management |
The 6 hour ‘golden period’
The earlier lavage and debridement is achieved, the greater the reduction in the bacterial load (Waldron, 2003). Infection risk is significantly lower as a result.
Wounds that are less than 6 hours old, once lavaged and debrided, may be closed directly assuming there is enough tissue available to do so (Bellah and Williams, 1999). This principle of a ‘golden period’ relies on the fact that bacteria are not likely to be in quantities greater than 105 bacteria/cm2 within this time with adequate lavage and wound preparation (Krizek and Robson, 1975). However, a decision to close at this stage should always include a full assessment of the extent of injury and any potential compromise that could lead to tissue breakdown (Table 5). Certain wounds will also be an exception to this rule due to the virulence of bacteria and mechanism of injury.
Antimicrobial dressings
There are several different dressings that can bew, or as a stand alone treatment in local wound infections.
Many of the more modern dressings offer a broad spectrum antimicrobial effective without antibiotics. Moist wound management should be maintained if possible and should not be forfeited in place of a more potent product. Personal preference may also dictate choice as many dressings are similarly effective in terms of antimicrobial effect (Table 6).
| Antimicrobial wound dressings | Benefits |
|---|---|
| Manuka honey e.g. Activon®, Algivon® (Dechra Veterinary Products Ltd) | Effective against a broad spectrum of common wound pathogens including meticillin-resistant Staphylococcus aureus (MRSA). Applied directly to infected and sloughy wounds it effectively debrides through osmosis. A reduction in inflammation is also a benefit. An absorbent secondary dressing will be required to account for the osmotic action |
| PHMB (polyhexamethylene biguanide hydrochloride.) e.g. Covidien AMD® range (c/o Chanelle Vet UK) | A safe, fast-acting and broad spectrum antimicrobial, providing activity against a wide range of bacteria including MRSA. Available in a wide range of dressings to suit the wound type |
| Povidone and cadexomer iodine, e.g. Inadine® dressings (Systagenix) | Iodine may be used as a lavage at 1:10 dilution or can be presented in dressing form. Each is antimicrobial and effective against a broad range of microbes including MRSA. However, caution should be observed as contraindications include: hypersensitivity to iodine, thyroid diseases, renal failure and renal insufficiency and not suitable for open wounds covering more than 20% of the body. Furthermore, iodine is deactivated by organic material in the wound |
| Silver containing dressings: | There are many versions of dressings available that contain silver in an antimicrobial form. Many of these dressings are effective against the common wound pathogens including MRSA. |
| Silver (and activated Charcoal), e.g. Meditek Silver® (Fabtek ltd - www.fabteksolutions.com); silver alginate, e.g. Silvercell® (Systagenix); nanocrystalline silver, e.g. Acticoat® dressings (Smith and Nephew) Silver sulphadiazine paste, e.g. Flamazine® (Smith and Nephew) | |
| The type of dressing should depend on its function in the wound and should be in contact with the wound bed to be effective. All silver dressings (except Flamazine®) need to be moist in order to have an antimicrobial effect. Exudate from the wound should be sufficient to achieve this. Acticoat is the exception and requires pre-moistening with sterile water. Flamazine® is a useful silver preparation that combines antibiotic in a form of silver sulphadiazine. It is frequently used for diffuse wounds, burns and awkward areas that may be difficult to dress otherwise. The patient will need to be managed so that the product remains in place and application may need to be frequent |
When infection is not the problem
It is easy to jump to the conclusion that the wound is infected because it is not healing as expected. Swabs and culture may reveal that there is bacterial colonization, but despite treatment and all the right preparation, a wound may still not progress. In these instances it is possible that there are other factors inhibiting healing that may have been forgotten or overlooked. These should be ruled out and treatment should be adapted to avoid or address any factors that are present so that the wound can progress to healing unhindered (Table 7).
| Movement at the wound site | Edges fail to unite and epithelium unable to adhere to wound bed |
| Foreign body | It is not always possible to find everything that entered the wound, an X-ray may be required |
| Necrotic tissue | Necrotic tendon and bone can provide a foreign body effect that will prolong inflammation preventing granulation and closure |
| Poor blood or oxygen supply | Tissue damage during trauma may be extensive and perfusion may be too low for healing. Anaemia may also prevent oxygen supply to local tissue |
| Poor nutrition or health | Hypoalbuminemia below 30 g/l significantly retards healing. Coexisting diseases should be ruled out |
| Local tissue issues: dead space tissue tension | Dead space will mean pocketing of exudate and potential healing delay. Wounds under excessive tension may repeatedly break down |
| Iatrogenic factors | General interference and use of inappropriate products |
| Cell transformation | Wounds failing to respond despite best efforts should be biopsied. Histological changes may be the problem |
Conclusion
There will always be wounds that become infected or present to the practice already infected. In these cases a plan is required that includes lavage, debridement of devitalized tissue, culture and sensitivity to identify an appropriate antibiotic if systemic management is required.
Management of the wound should consist of choosing the appropriate dressings to deal with any remaining local wound infection while supporting a healthy wound environment. In the presence of systemic infection dressings alone are not sufficient and a systemic antibiotic must be selected based on culture sensitivities.
Recognition and minimization of the factors that can contribute to healing delay and sustain or support infection will ensure everything is being done to maximize the chances of a positive outcome.