Surgical site infections (SSI) are one of the most common causes of morbidity in the surgical patient despite preventative methods and use of prophylactic antibiotics to reduce the incidence in humans and in veterinary patients (Melling et al, 2001; Eugster et al, 2004). Normal, intact skin prevents microorganisms entering the body by two mechanisms: intact epidermis prevents physical passage of microorganisms; and cell-mediated immunity and production of antibodies prevents microbial invasion (Dohmen et al, 2006). The skin's flora exists on the host skin in a very delicate and complex ecosystem which is disrupted at surgery along with the body's immune system, which lowers the body's natural defense barriers.
Recently there has been further concern regarding SSI due to multi-drug resistant (MDR) bacteria emerging as veterinary and zoonotic pathogens, e.g. meticillin-resistant Staphylococcus aureus (MRSA) (Weese et al, 2006). In a recent human study, mortality rates following cardiac surgery were as high as 74% due to mediastinal infection with MRSA (Dohmen et al, 2006). It is important to prevent infection of the patient and to prevent yourself and other co-workers becoming colonized with these MDR bacteria.
Control of infection in the operating theatre
The type of surgery being performed will influence the infection control procedures performed and the effectiveness of controlling infection in the operating theatre. Surgeries can be classed as refined clean, clean, clean-contaminated, contaminated and dirty (Moore, 1995). For example, lancing of a cat bite abscess would be a dirty surgery as would debridement of a dirty wound on a horse's leg. Elective orthopaedic procedures such as chip fragment removal from a metacarpophalangeal joint would be classed as a refined clean surgery. Colic surgery in the horse would be classified as a clean-contaminated surgery if the intestine is entered but there is only minor spillage.
The use of implants (orthopaedic devices, prosthetic meshes) in surgery can also increase the risk of infection due to the presence of a foreign material (Moore, 1995).
Cleanliness and preparation of the patient
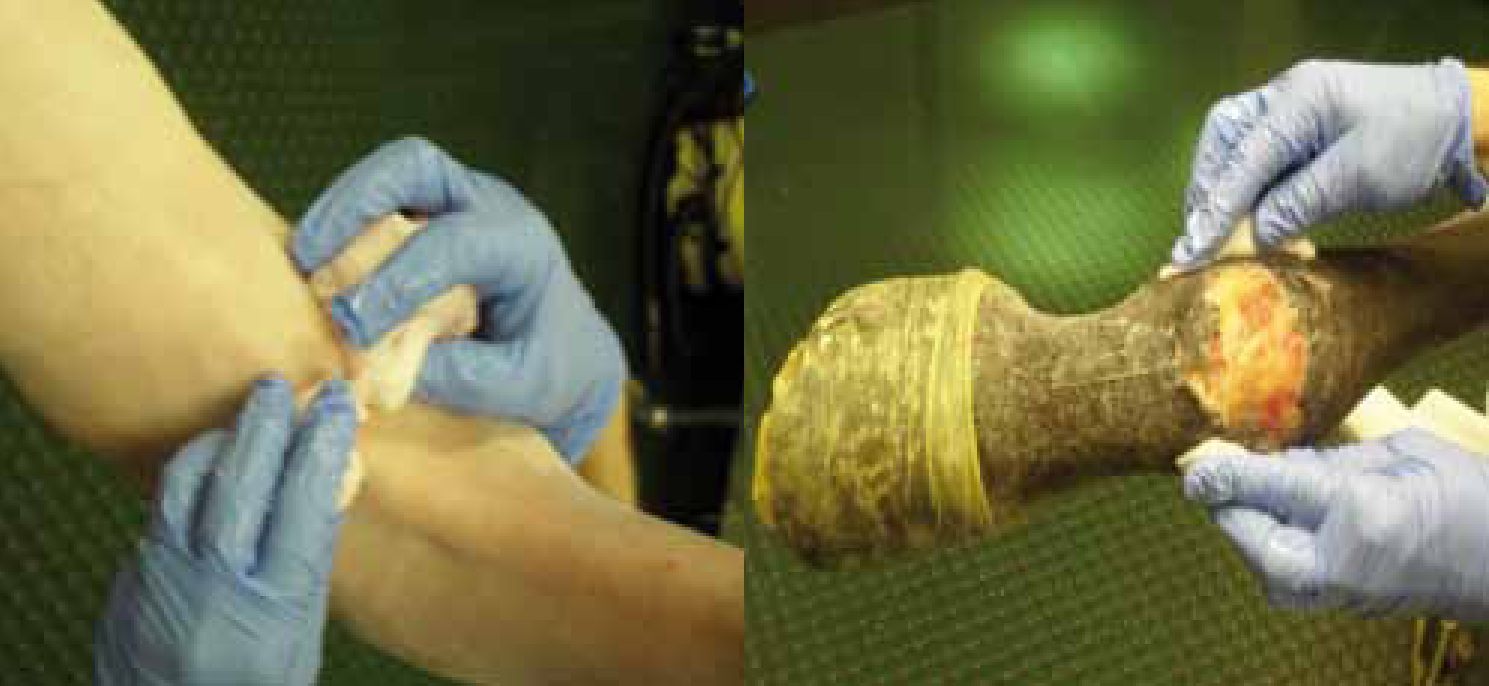
The patient is usually the primary source of pathogens involved in SSI (Baines, 1996; Dohmen et al, 2006). Many studies have looked into the removal of hair and bathing of humans prior to surgery with chlorhexidine to reduce the incidence of SSI (Tanner et al, 2006; Dizer et al, 2009; Mimoz, 2010). Removal of hair with clippers helps reduce SSI compared with hair removal by shaving (Tanner et al, 2006). Controversy remains regarding whether the risk of SSI decreases if hair is removed by clippers the day before surgery or just prior to surgery (Tanner et al, 2006; Dizer et al, 2009). It is ideal to clip the patient in a preparation area adjacent to the theatre to avoid contamination of the environment in theatre with hair and dirt particles. This is often possible in small animals where moving the patient into theatre after induction of anaesthesia is relatively easy, compared with moving a larger patient such as a horse. In equine surgery, often a preparation area is not available for clipping once general anaesthesia is induced. If cliping has not been possible prior to surgery then this should be performed in theatre with care to remove as much dirt, hair and debris as possible with a vacuum cleaner. Careful aseptic preparation of the surgical site is essential (Figure 1a and 1b). Cleaning with chlorhexidine-alcohol is superior to cleaning the skin with povidone-iodine to prevent superficial skin infections and deep incisional infections (Chaiyakunapruk et al, 2002; Darouiche et al, 2010). However povidone-iodine is still used successfully in the preparation of ocular surgery and mucous membranes, where chlorhexidine can cause damage to these delicate tissues (Wu et al, 2006).
During general anaesthesia patients are unable to regulate their body temperature (Sesslr, 2000). Keeping the patient warm during general anaesthesia has been shown to reduce incidence of SSI (Melling et al, 2001). In small animals body temperature can be maintained using heat pads covered by towels or blankets and covering the body with bubble wrap. In equine surgery it is not usually necessary to cover the horse with insulating material provided the temperature in the operating theatre is not lower than normal room temperature. In the authors' opinion foals should be kept insulated as in small animal theatre, as they have a larger body surface to mass ratio, and therefore lose heat more rapidly. Commercial warming products are available which the authors find very useful in the hospital (Bair Hugger Therapy, Arizant Healthcare Inc. USA). With all warming devices it is important to follow manufacturers' instructions and never place an animal directly onto a heat pad or hot water bottle as skin burns can occur, which can be very serious for the patient.
Dress of personnel in the operating theatre
People entering an operating theatre should be in a change of clothes from their normal work clothes, i.e. scrubs. These should be laundered after each use in theatre or after contamination with organic debris. Scrubs used in the operating theatre should be retained for use in theatre only and not used for consults or worn outside of work (Figure 2, 3). This helps reduce the risk of carrying microorganisms into the clean operating environment. The use of surgical facemasks by the operating team has not been shown to prevent SSI in human or veterinary science; however their use is common and may prevent infection from the patient to the operating team (Bahli, 2009; Webster, 2010). Surgical hats should be worn by the operating team to prevent debris and microbial organisms contaminating the surgical site (Figure 4). Fingernails should be short and forearms should be bare (Napolitano, 2006) with no jewellery (only a plain wedding band is acceptable in the authors' hospital). The most common mode of transmission of pathogens is via the hands and good hand hygiene is the most important factor in reducing nosocomial infections within hospitals (Napolitano, 2006).
Personnel entering or leaving the theatre
In human hospitals much work has been done to assess the effect of personnel entering and leaving the operating theatre during surgery. It has been shown that not only does movement of theatre staff impact the particulate levels in the air but also causes an increase in surgical mistakes, which is not advantageous to any situation (Young and O'Regan, 2010). Therefore, movement in and out of theatre should be limited to emergency/essential movement only. It has been shown that bacteria from personnel on the peripheral field of the operating theatre, with no physical contact with the patient, have been isolated in the surgical site (Gosden et al, 1998).
Equipment cleaning, disinfection, sterilization
An operating theatre should be a clean, restricted personnel zone. It is important that equipment is cleaned first, then either disinfected or sterilized appropriately prior to being stored correctly. Before equipment can be disinfected or sterilized, it is essential to clean the equipment. Cleaning is the physical removal of organic debris from a surface. Disinfection is the inactivation or killing of microbes, however not all microbes (in particular spores) are killed by all disinfection solutions. Various commercial disinfectants are available which should be used in accordance with the manufacturer's guidelines (Safe4, Safe Solutions, Cheshire, UK; Trigene, Medichem Int, Kent, UK). Some do guarantee the destruction of all organisms, however correct strength of disinfectant which is used for the appropriate length of time is essential (follow manufacturer's directions carefully). The anaesthetic equipment should be cleaned on a routine basis according to the manufacturer's directions. Pseudomonas aeruginosa is a common opportunistic microbe which can be passed from patient to patient if anaesthetic equipment is not disinfected regularly (Ichikawa, 2010).
Sterilization is the complete killing of all life forms from surfaces, including spores. Sterilization usually is performed in a pressure vessel called an autoclave. High temperatures and steam are used to kill all microorganisms on equipment. Packaging of surgical kits is important as packing instruments in crepe paper followed by sterilization in an autoclave results in a longer shelf life when stored in a closed cabinet (Figure 5). Surgical kits packaged in heat-sealed paper and transparent plastic pouches remain sterile for up to 1 year even if not stored in a closed cupboard (Freeman, 2006). The date on which the equipment was sterilized should be written on the packaging.

Ethylene oxide (EO) is a gas often used to sterilize equipment. It penetrates packaging rapidly and can be used to sterilize most equipment that is not suitable for sterilization by high temperatures and steam. EO can damage tissues if it is left on the equipment, therefore equipment must be aerated after EO sterilization (Freeman, 2006).
Cold sterilization is the term commonly used when equipment is not suitable for sterilization in an autoclave, but is disinfected in cold solutions of chemicals. Cold sterilization really only disinfects equipment, however, some manufacturers guarantee sterilization after the equipment has been soaked in the chemicals for a specified length of time (Baines, 1996). It is important to leave the equipment in the sterilization product for the sufficient amount of time to ensure it is disinfected (or sterilized) correctly.
Cleaning and disinfecting the floors and surfaces
Environmental surfaces are not often associated with SSI, however, they should still be cleaned and disinfected on a daily basis, if not more regularly, and between patients. If visible soiling with bodily fluids is present, this should be cleaned immediately and disinfected with hospital disinfectant (Safe4, Trigene). It is important to remember that cleaning is the physical removal of organic matter from a surface whereas disinfection is the killing/inactivating of all microbes on a surface (follow manufacturer's directions carefully).
Monitoring of infection levels
Clinical audit to record the incidence of SSI is essential to monitor whether preventative methods are working. A sudden increase in incidence of SSIs may indicate if there is a problem with adherence to protocol or a fault in the process of sterilization which may otherwise have gone undetected.
Conclusion
Contamination of the surgical site can potentially arise from the patient, the surgical team, the operating theatre and surgical equipment. The principles behind infection control in the operating theatre are to try to limit contamination of the surgical site with microorganisms from these places. Obvious differences between humans and domestic animals (e.g. hair coat and hooves) mean that direct extrapolations from the human literature are not always possible, however, much of the clinical evidence-based research from the human medical field can be used to improve our understanding of ways to manage infection control in the operating theatre.
