This article provides veterinary nurses with an introduction to the set up and management of a veterinary dispensary and helps identify the differences between requirements that are desirable and those which are legally necessary or specified by professional or organizational bodies such as the Royal College of Vetereinary Surgeons (RCVS), Veterinary Medicines Directorate (VMD) or British Small Animal Veterinary Association (BSAVA). It provides reasons for having an efficient and effiectively run dispensary and one that complies with relevant legislation and regulations, together with the key points to help with day-to-day management of the veterinary dispensary. It is not within the scope of this article to include information on online pharmacies or setting up a business from your home. Although some of the information provided will be similar.
The role of the veterinary nurse (VN) is expanding and veterinary practice is becoming increasingly more specialized. These changes offer VNs opportunities to diversify and specialize their skills and become actively in-volved in the veterinary dispensary. However, since April 2011, registered VNs have become accountable for their actions. This means that it is essential that VNs understand and comply with the legal requirements and professional codes that are involved in managing medications within the veterinary practice. Failure to do so could result in disciplinary action.
An essential point to highlight at this stage is the difference between the terminology of pharmacy and dispensary. A pharmacy typically requires a pharmacist to be on duty at all times when the pharmacy is open. Pharmacists are governed by the Royal Pharmaceutical Society. A pharmacist has expertise in medicines and is able to offer healthcare advice. The premises of a pharmacy are inspected by the General Pharmaceutical Council. A dispensary is the term which may be best applied in the vast majority of veterinary practices; it is unfeasible or unnecessary to employ a pharmacist to dispense the array of veterinary medical products. In many cases, the majority of dispensing is performed by VNs on request from the veterinary clinician. Veterinary dispensary inspections fall under the remit of the RCVS, but are dependent on type of registration as to inspection criteria involved.
Registration of practice premises
Veterinary practices that hold medications for supply purposes must be registered with the RCVS. The veterinarian must also be registered with the RCVS to prescribe medications. There are two options of registration and inspection:
The register of practice premises will be held by the RCVS on behalf of the secretary of state (Veterinary Medicines Directorate, 2011b).
Design and layout
The storage of medications should be best positioned for the running of the practice and will vary from practice to practice and is somewhat dependent on the structure of the building. Ideally, a central location which is accessible for staff is beneficial, but which is not accessible to the general public. The BSAVA guidance on premises licence and inspections specify that Prescription Only Medicine-Veterinary (POM-Vs) must not be of self service (British Small Animal Veterinary Association, 2012a). This means it is unacceptable to leave drugs unattended in the consulting room or out in the waiting room in reach of clients to handle or potentially misuse. All medications are best located in the central controlled dispensary or kept locked in a cupboard in the consulting room. In the first instance, the following questions may help VNs appraise the current system used within their practice:

When working on the layout and how the dispensary will be utilized, it is essential to keep in mind health and safety issues.
Safety in the dispensary
The BSAVA website has a section on health and safety in the dispensary (British Small Animal Veterinary Association, 2012b). The following are some useful points to consider:

Storage and stock control
All medications MUST be stored according to manufacturer's recommendations, found on the Summary of Product Characteristics (SPC) (British Small Animal Veterinary Association, 2012c; Royal College of Veterinary Sureons, 2012).
Temperature control is an essential requirement where medications are stored. Minimum and maximum temperature must be recorded and available for staff and inspectors to view. There are options available on keeping records: digital thermometers are useful and temperatures can be recorded daily by pen and paper, or by electronic devices which are able to store data and transfer them via a software package onto the computer. The fridge temperature for medications will need to be set between 2–8°C. A SOP MUST be in place for temperature recording and there should be a designated person as a point of contact for monitoring temperature control and to resolve temperature-related issues. If this person is away the SOP must explain who else can monitor the temperature recordings and action against a fault recorded. It is advisable to contact the manufacturer of the medication if there are any concerns that the product was exposed to extreme temperatures. The manufacturer will advise best practice, which may mean discarding the product. This is an added expense to the practice set budget. Recording of expenditure wastage is good practice. If the practice attends house or farm visits the car must be fitted with suitable control measures for temperature recording of medications and lockable units. It is advisable to remove all medications over night or when the vehicle is not in use.
Medicines should be stored away from excessive light and moisture. The dispensary should be clean and tidy with secure shelving.
An SOP should be in place and visible for stock control purposes. When stock is received it should be checked for damage and expiry dates. Expired or damaged stock MUST be kept separate from in use/sale stock. These products can be returned to your wholesaler or recorded and disposed of in the pharmaceutical waste bin. New stock should be placed behind older stock on the shelf (expiry dates should be checked).
Batch numbers MUST be recorded when stock is received, either on paper or electronically. On first opening of medications the batch number requires recording. For non food producing animals each batch does not need to be recorded to the patient's name but records MUST make it possible to track patients who received that batch. The author advises as good practice recording all batch numbers at time of dispensing to patient records. The practice management system will have the capacity to do this; it is advisable to get to know the practice management system and record all stock onto the system with their batch numbers, expiry dates and quantities. The total quantity in stock should be accurate, but checked frequently for any malfunctions and if discrepancies are observed they should be investigated. In the event of a batch recall an efficient stock control system will allow drugs, which have been dispensed to patients, to be viewed.
An annual audit is required as part of RCVS inspections (British Association of Small Animals, 2012d). Records must be available of all incoming and outgoing transactions of stock held.
Flammable medicines should be stored in a separate cupboard clearly labelled and near to ground level to prevent accidental breakage. When loose tablet pots and blister packages are opened, they should be labelled with the date of opening and for liquids and injecta-bles, with the date of expiry for discarding.
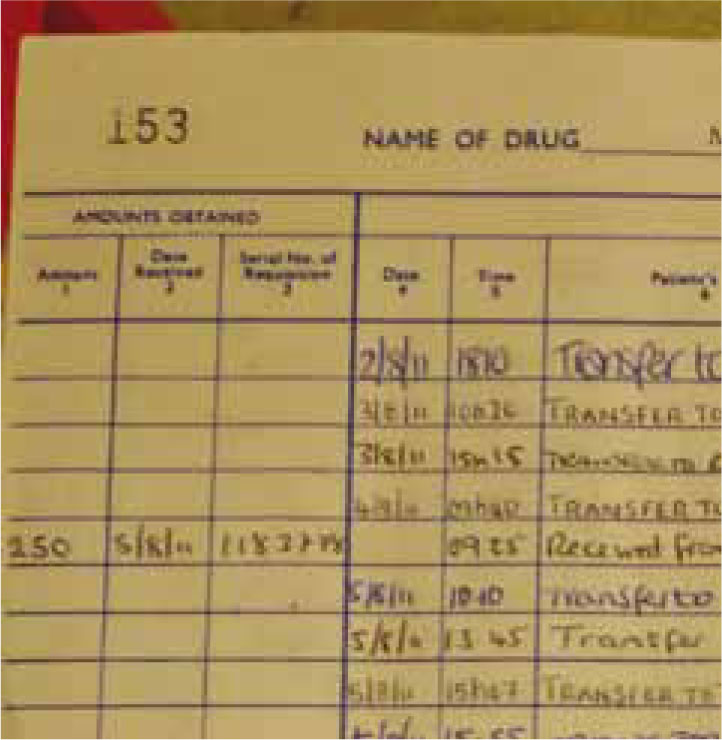
Schedule 2 drugs must be recorded on arrival into the controlled drugs book and locked in a cabinet secured to the wall. Schedule 3 drugs and ketamine should also be locked in the controlled drugs cabinet. Only a select few people should have access to the controlled drugs cabinet. When removing stock to another ward or to give to the patient this must also be clearly written in the drugs book (Figure 3). Controlled drugs books must be kept for a minimum of 2 years after the last date of entry. It is also advisable to keep a controlled drugs book for ketamine.
The premises holding medications must be of a secure permanent building free of vermin, and an area where hand washing can take place. There must be no consumption of food and drink in the dispensary (Veterinary Medicine Directorate, 2011b).
Supply of and dispensing medicinal products
In order to prescribe medications in category POM–V, the veterinary surgeon must have carried out a clinical assessment (British Association of Small Animals, 2012e).
A SOP must be in place for a veterinary surgeon to authorize that they are satisfied for a staff member to hand over the medication to the client and be confident that the staff member will inform the client of how to give the medication and any warnings; any contraindications must be interpreted. The staff member must feel confident that the client is competent to give the medication for their pet's treatment safely and only for the prescribed use.
Medications dispensed must be in their authorized packaging contained in Marketing Authorization. Only the veterinary surgeon, pharmacist or a suitably qualified person (SQP) can break open packaging for dispensing. Only a veterinary surgeon or pharmacist may break immediate packaging for dispensing. A packet insert or SPC must be provided with every medication dispensed.
A packet insert or SPC can be obtained from the medication box and photocopied, or printed online from the manufacturer's database, or obtained online via the VMD (http://www.vmd.defra.gov.uk/ProductIn-formationDatabase/) or BSAVA site. A veterinary surgeon will need to register online; this site also offers SPC for products used of license. The cascade must always be followed (see notes later). SPC for human drugs under the prescribing cascade can also be found on www.medicines.org.uk/emc.
Loose tablets must be placed in a light sensitive amber tablet pot/bottle usually plastic with a child proof safety lid. Liquids must be dispensed in a light-sensitive amber glass bottle with a child proof safety lid. Blister packets may be supplied in an envelope or cardboard tablet box. Cytotoxic blister packs are best placed in a plastic pot with child proof safety lid. Cyto-toxic warnings are best written on the label. All medications must be clearly labelled with:
Labels must not cover important information on the manufacturer's packaging. Medicines returned by clients should be disposed of as there is no guarantee on the conditions in which they have been stored (Veterinary Medicines Directorate, 2011b).
Written prescriptions
Veterinary surgeons will often be asked by clients to write a prescription for their medication to buy it cheaper elsewhere (Figure 4). The veterinary surgeon cannot refuse to do this but must explain to the client that there will be a a fee for the written prescription service.

The Competitions Commission request that veterinary practices must advertise to clients that written prescriptions are available on request (British Small Animal Veterinary Association, 2012b). Veterinary practices do have the right to charge for the prescription. This charge is entirely at the discretion of the practice, but a sensible fee is advisable. The veterinary practice must also display for the general public to see the top 10 licensed medications sold within the practice as well as indicate that clients have the right to ask the price of any drug on request.
More information on written prescriptions can be found at www.bsava.co.uk. The following is useful information:
Pharmacovigilance
Any drug adverse reactions must be reported as requested by the VMD. A member of staff must take responsibility for this role and make sure all reports are sent to the VMD. An adverse reaction is anything that results from the medication that has caused a reaction of some kind to the patient and which may or may not have had an effect on the patient's treatment regimen. Giving an injection or oral medication in practice enables a practitioner to observe a reaction and respond accordingly.
The VMD have an online Suspected Adverse Reaction Surveillance Scheme Form. The form is yellow and all adverse reactions must be reported on this. Anyone can fill in the form, if access online is not available the form can be found in the back of the NOAH compendium; all forms must be sent to the VMD. It is advisable to keep a copy for your records and a legal requirement to have a designated member of staff to monitor pharmacovigilance (Veterinary Medicine Directorate, 2011b).
Waste regulations
Once a month it is good practice to check all stock for expired medicines. Most injectables once opened have an expiry date of 28 days. It is against the law to dispense medications past their expiry date.
The following waste regulations are set out for England and Wales. For further guidance on waste contact the environment agency or British Veterinary Association (www.environment-agency.gov.uk and www.bva.co.uk).
Pharmaceutical waste
All pharmaceuticals disposed in the pharmaceutical waste bin must be recorded and available for contractors or inspectors to view. All tablets discarded must be kept in their blister packets or kept in pill pots. It is advisable to keep liquids and tablets separate due to possible chemical reactions.
All out of date schedule 2 drugs must be denatured before disposal; disposal is by emptying the contents of the vial onto absorbent material, i.e. cat litter or incontinence pads, and then disposing of it in the pharmaceutical waste bin. A witness is required to be present at this time and sign the controlled books to show this has taken place. The witness must be a police controlled drugs liaison officer, member of the Inspections and Investigations Team (IIT), Inspector of the RCVS practice standards scheme or a veterinary surgeon who is independent of the practice.
Schedule 3, 4 and 5 drugs require denaturing and as with schedule 2 drugs recorded on the pharmaceutical disposal log sheet and controlled books if present. Two members of staff should be made available to witness and sign the denaturing process (as for schedule 2 drugs).
Pharmaceutical waste should be disposed of in leakproof containers, usually green bins but dependent on the contractor. Nurses are advised to discuss appropriate containers with them — EWC 18 02 08 (British Veterinary Association, 2012).
Cytotoxic and cytostatic waste
The following are classed as hazardous waste and carry variable codes:
These drugs must be disposed of by specialist contractors. They fall under the EWC coding system of 18 02 07.
Items for disposal include contaminated syringes, needles, unused medicine, contaminated protected clothing, cannulae, and residue in vials.
The medicines in this classification are chemotherapeutics, antiviral medicines, ciclosporin, and hormonal preparations.
This waste must be contained in PURPLE containers. Sharps and pharmaceutical bins must have purple lids and waste bags must be purple or yellow with a purple stripe and have purple ties. The codes must be clearly identifed on each item. All waste collected by the contractor must be recorded on a consignment note and signed by both the contractor and the practice. These records must be kept for 3 years.
Healthcare/clinical waste
Needles must be placed in British standard sharps bin. The bins must contain the following EWC code 18 02 02. These bins are yellow with yellow lids.
Clinical waste is hazardous waste which consists of: animal tissue, blood or other body fluids, excretions, drugs or other pharmaceutical products, swabs and dressings, syringes and needles. This waste is identified as waste which may cause infection to any person or animal coming into contact with it. It should be disposed of in yellow or orange bags or containers with EWC code 18 02 02.
Photographic chemicals to be disposed of in separate fixer and developer containers. EWC code 09 01 01 — developer, and 09 01 04 — fixer.
Offensive waste
Veterinary waste that is not clinical waste but may be unpleasant to the senses is offensive waste. It must not present risk of infection to a person or another animal if they come into contact with it. It includes: animal bedding; gloves; masks; paper towel etc. It should not include blood or contaminated bedding, clothing etc — EWC code 18 02 03. This type of waste should be disposed of in a yellow bag with black stripe.
SQPs
The Animal Training Regulatory Authority (AMTRA) qualification is available for those persons with an interest in dispensing. Table 1 shows the different types of qualifications a SQP can obtain and the CPD points that can be achieved.
| SQP Type | Modules | VPS medicines supplied | CPD Points |
| R-SQP | FAM+EQM+CAM | All VPS Medicines | 80 points |
| G-SQP | FAM+EQM | VPS-farm animals and equines only | 60 points |
| K-SQP | FAM+CAM | VPS-farm and companion animals only | 60 points |
| E-SQP | EQM+CAM | VPS-equines and companion animals only | 50 points |
| L-SQP | FAM | VPS-farm animals only | 50 points |
| J-SQP | EQM | VPS-equines only | 30 points |
| C-SQP | CAM | VPS-companion animals only | 30 points |
FAM – Farm Animal Module; EQM – Equine Module; CAM – Companion Animal Module
There is now a new module, A-SQP = avian (poultry and pigeons). This module can be combined with companion animal, equine or both.
The exam consists of a base module containing legislation and then questions on the relevant section of the modules to be taken and a viva exam. For more information on the conversion exam visit www.amtra.org.uk The AMTRA website is very informative.
What medicines can SQPs prescribe and/or supply?
An SQP is entitled to prescribe and/or supply the categories of product that fall within the scope of the qualification they have obtained and the registration they hold. Each SQP follows a code of practice set out by the VMD and distributed by AMTRA.
The ranges of veterinary medicines available to SQPs fall within the following distribution classifications:
For companion animals, an SQP can dispense some ectoparasite and endoparasite treatment.
Active ingredients that are available over the counter are:
There are a few different product brands from pharmaceutical companies to each active ingredient indicated above.
Questions to ask clients
It is best practice to always ask clients a few questions to identify what treatments their pets require. This helps build a relationship between the VN and the client and encourages them to come back for more advice.
A list of questions can be devised to use as part of a nurse consultation. This will rule out discussions in the waiting room in front of other clients creating less distractions and allowing the client to feel more comfortable talking about their pets to identify which flea or worming products they require. The following questions are relevant to the SQP who has gained a qualification to prescribe POM –VPS and NFA – VPS medications:
An SQP in practice can make a difference:
Cascade
In brief the cascade is to prevent potential harm to an animal caused by an unlicensed medication. VNs and SQPs do not have the right to prescribe unlicensed medications.
The veterinary surgeons have the responsibility to decide on the medication the patient requires. A licensed animal product should always be the first choice to treat the patient. If there is not a licensed product available for the specific species a check should be made to see if the medication is licensed for another species which may be suitable. If not the veterinary surgeon can choose a human drug at his/her discretion. Consent must be obtained from the client.
Conclusion
VNs handle medications on a daily basis, it is important to not only understand why a particular type of medication is being used to treat patients but how to correctly dispense and discard medications.
With updates on a yearly basis from the VMD on medicine regulations it is important for VNs to understand the process of drug management, from how medicines are manufactured and licensed to the end prescribing and dispensing process. The VN in practice can utilize their skills monitoring stock control, budgets, waste and pharmacovigilance and feel confident in doing so.
