There are approximately 9000 known species of reptile (Rendle and Crack-nell, 2012). These are subdivided into four categories:
Reptiles can be difficult to clinically assess and a blood sample can be an important diagnostic step. A blood profile may include:
This article will focus on blood-sampling techniques in Chelonia and Squamata.
Reptiles have a different cardiovascular system to that of mammals as their heart has three chambers, two atria and one ventricle. It functions predominantly in the same way as a four-chambered heart. The ventricle has three sub-chambers and receives blood from the right and left atrium (Aspinall and Cappello, 2009). Reptiles have a renal portal system which directs blood from the tail and hind limbs to the kidneys. Therefore, drugs that have been injected into the caudal aspect of the body may be directed and excreted through the kidneys before reaching the systemic circulation (Aspinall and Cappello, 2009). However, recent evidence suggests that this may not be of clinical importance and therefore needs further evaluation.
Blood collection
There are several aspects to consider when taking a blood sample from a reptile:
Chelonians
Sites which are commonly used for venepuncture in Chelonia are:
Other sites include:
| Biochemistry | ||
|---|---|---|
| Albumin | g/litre | 13.0–26.0 |
| Total protein | g/litre | 23.0–44.0 |
| Uric acid | Umol/litre | 124–446 |
| AST* | Iu/litre | 0.00–210 |
| Creatine kinase | Iu/litre | 0.00–2620 |
| Urea | Mmol/litre | 0.00–2.10 |
| Calcium | Mmol/litre | 1.65–3.30 |
| Phosphorous | Mmol/litre | 0.48–1.81 |
| Haematology | ||
| White cell count | 10^9/litre | 1.0–4.5 |
| Haemoglobin | g/dl | 6.4–15.7 |
| PCV** | % | 17–42 |
| Heterophils | 10^9/litre | 0.50–5.00 |
| Lymphocytes | 10^9/litre | 0.27-2.50 |
| Monocytes | 10^9/litre | 0.00–0.20 |
AST: aspartate aminotransferase
PCV: packed cell volume
Jugular vein
The jugular vein (Figure 1) lies laterally and superficially on the neck. Jugular venepuncture should be attempted first as this sample is least likely to be diluted with lymph (Barrows et al, 2004). A 5/8-inch 23-25 gauge needle and the smallest syringe possible should be used.
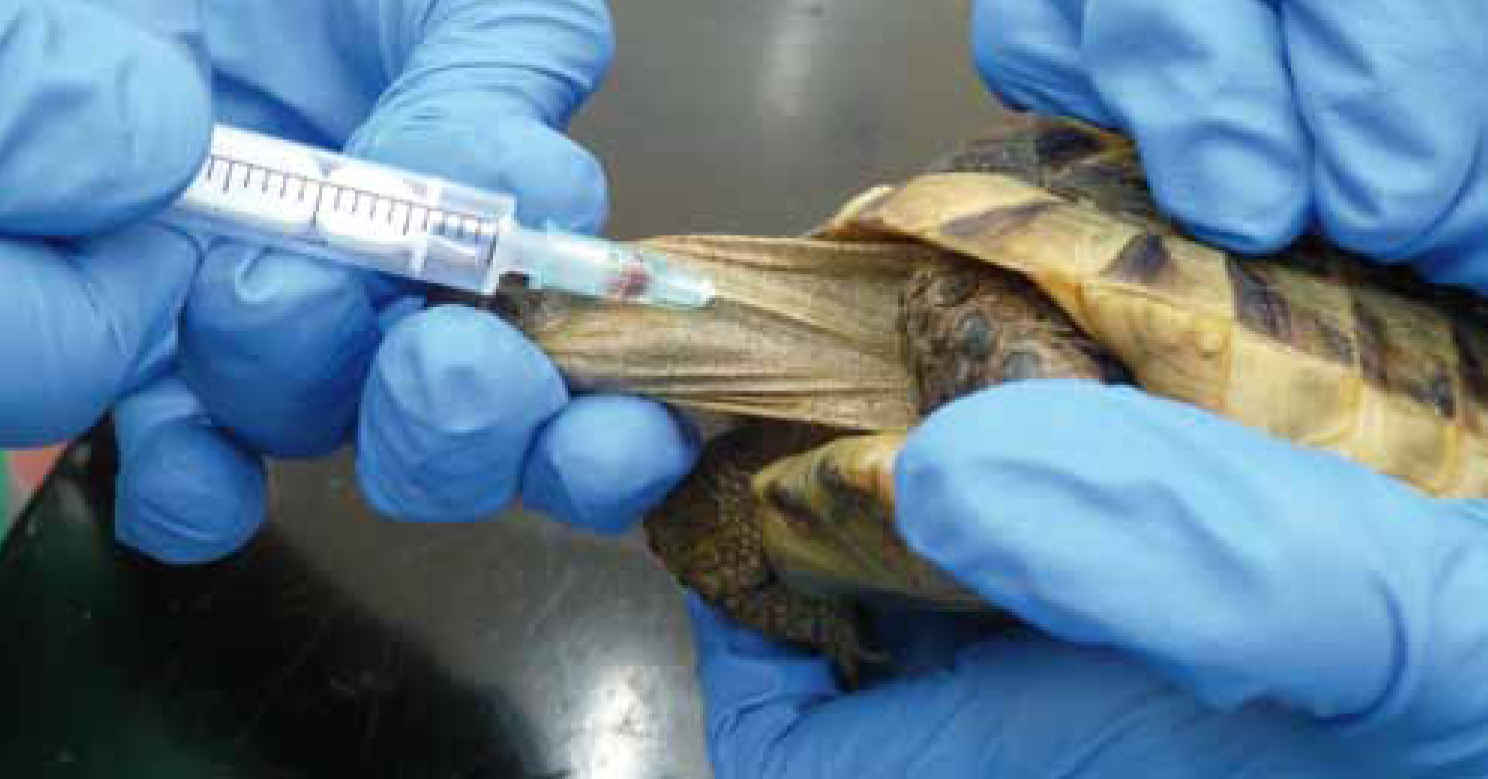
Restraint of the Chelonian may require sedation or anaesthesia. In the ill or easily restrained patient, this may be possible with the help of an assistant. The animal is held in lateral recumbency with the neck extended to visualise the vein. To extend the head, gentle pressure is applied behind the mandibles. The forelimbs should be restrained by retracting them caudally, taking care not to exert excessive force as this could result in iatrogenic fractures. The assistant should place pressure on the thoracic inlet to facilitate a better visualisation; in the smaller Chelonian, this can be achieved by using a cotton bud so as not to restrict the view of the vein (Heard et al, 2004).
Supravertebral (subcarapacial) vein
The supravertebral vein (Figure 2) lies midline on the ventral aspect of the carapace, dorsal to the cervical vertebrae. A 1-inch 23-25 gauge needle and the smallest syringe possible should be used.
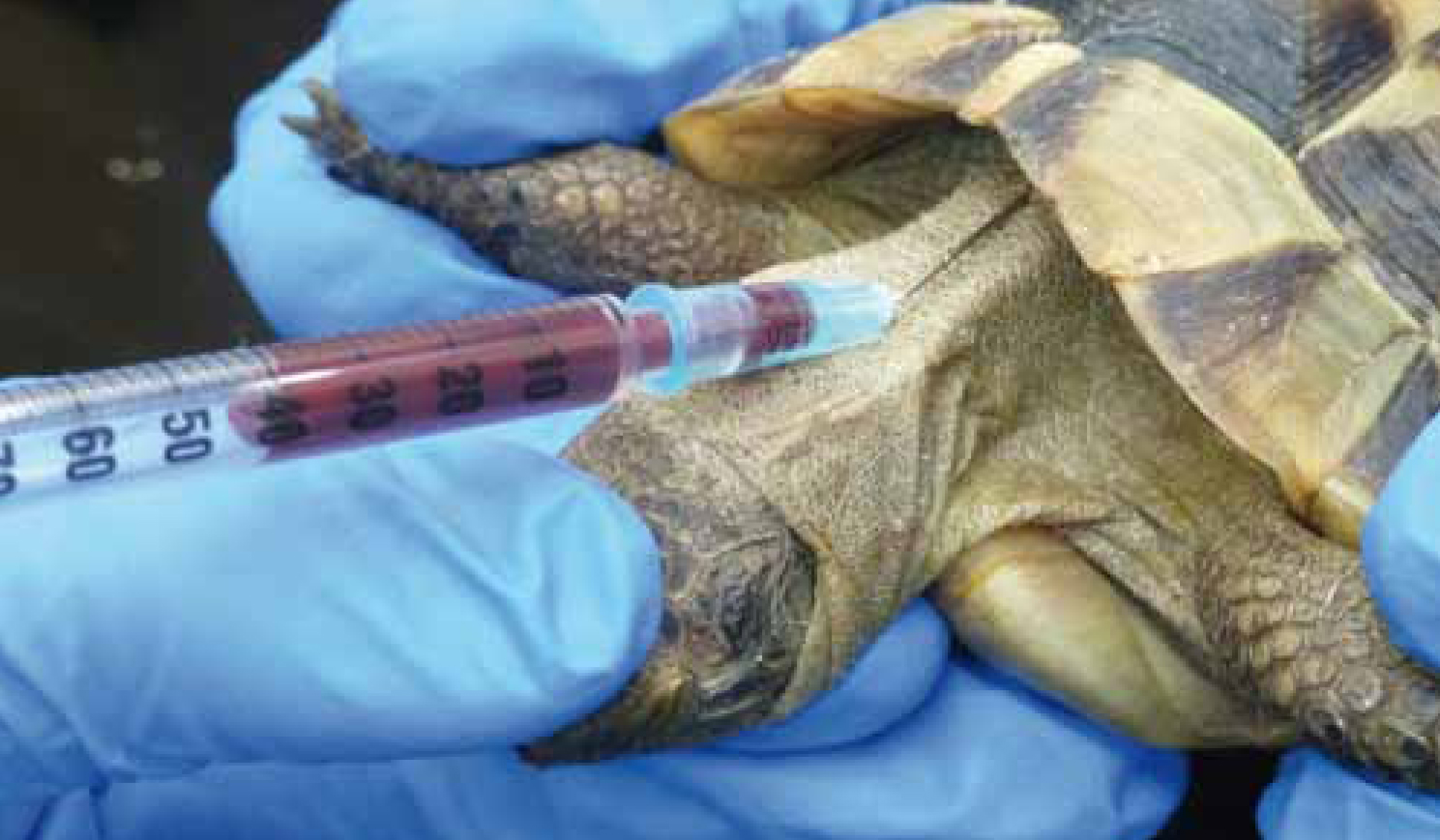
The needle is inserted where the skin meets the shell and advanced caudally at a 60° dorsal angle. Negative pressure is applied gently until blood has been aspirated. If the carapace is reached, the needle should be withdrawn slightly, then redirected and advanced while still applying negative pressure (Heard et al, 2004). Lymphatic vessels in this region increase the risk of lymph dilution.
Dorsal coccygeal vein
The dorsal coccygeal vein (Figure 3) is found on the midline aspect of the tail, dorsal to the vertebrae and is easier to locate in the larger Chelonian. A 5/8-inch 21-25 gauge needle and the smallest syringe possible should be used.
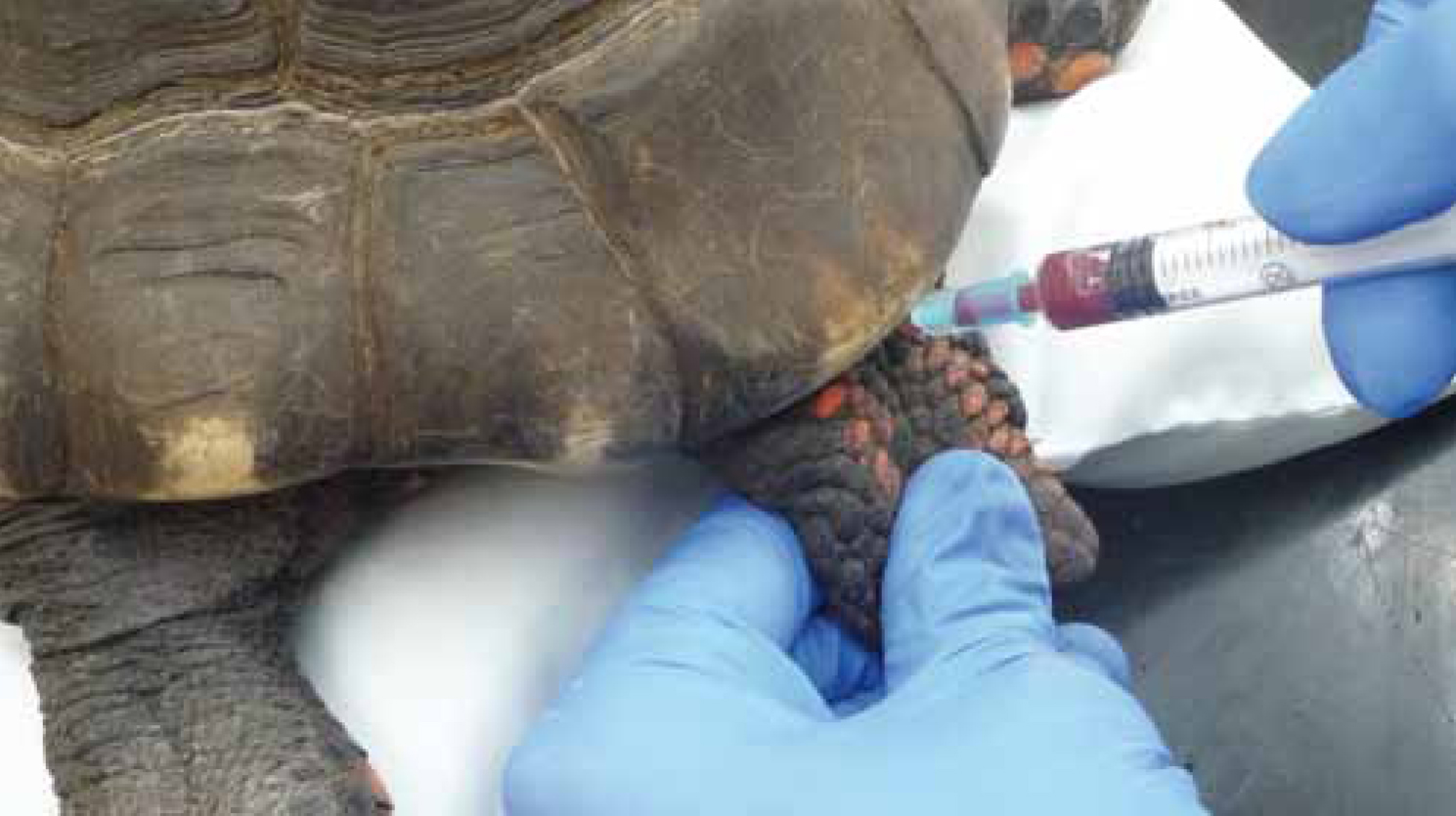
The needle is inserted slowly at a 45° angle into the midline at the junction where the tail meets the carapace (Barrows et al, 2004). It is advanced until the vertebrae have been reached and then slowly withdrawn while applying gentle negative pressure until blood has been aspirated. This area must be cleaned thoroughly to prevent faecal contamination (Heard et al, 2004). Lymphatic vessels in this region increase the risk of lymph dilution.
Squamata — Sauria
Venepuncture sites which are commonly used in Sauria are:
| Biochemistry | ||
|---|---|---|
| Albumin | g/litre | 25–35 |
| Total protein | g/litre | 52–72 |
| Uric acid | Umol/litre | 0–350 |
| AST | Iu/litre | 0–80 |
| Creatine Kinase | Iu/litre | 0–550 |
| Urea | Mmol/litre | 0.0–1.4 |
| Sodium | Mmol/litre | 140–160 |
| Calcium | Mmol/litre | 2.2–3.4 |
| Phosphorous | Mmol/litre | 1.1–2.3 |
| Haematology | ||
| White cell count | 10^9/litre | 1.5–8.5 |
| Haemoglobin | g/dl | 7.7–12.0 |
| PCV | % | 17–35 |
| Heterophils | 10^9/litre | 1.6–5.0 |
| Lymphocytes | 10^9/litre | 0.2–4.0 |
| Monocytes | 10^9/litre | 0.042–2.204 |
Poor handling of certain Sauria species can result in autotomy, a defence mechanism whereby the tail is shed at natural fracture planes (Maclean and Raiti, 2004) (Figure 4). The tail may take many months to regrow and will never have the same scale or colour formation as the original (Raftery, 2004). In some cases, the tail may never regrow; therefore, gentle handling with little pressure placed on the tail is advised.

Ventral coccygeal vein
The ventral coccygeal vein (Figure 5) lies on the midline ventral aspect of the vertebrae and is the preferred and most effective site for blood sampling.
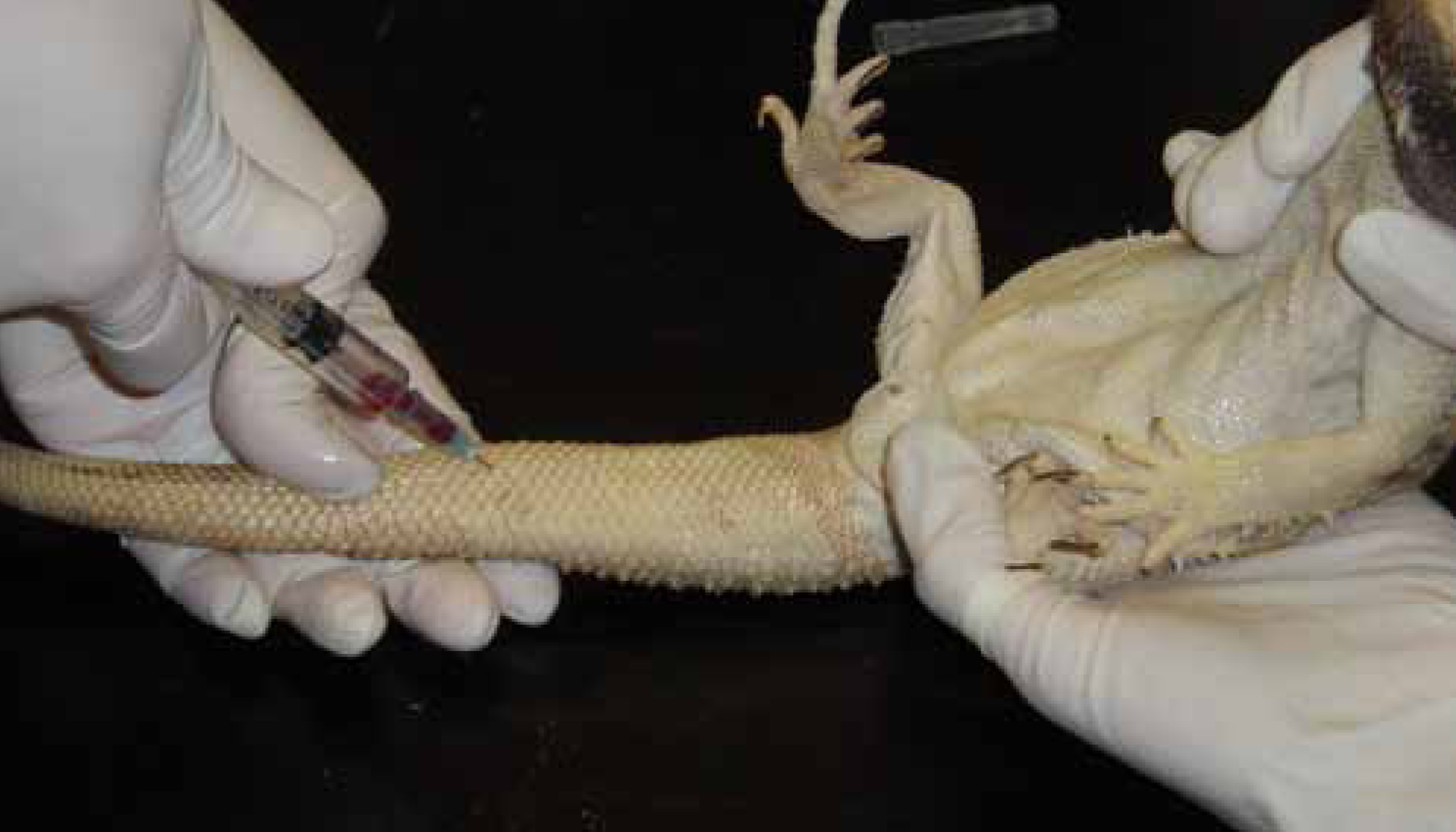
In small lizards, a 5/8-inch 23-35 gauge needle and the smallest syringe possible should be used. In medium-to-large lizards, a 1-inch 25-gauge needle should be used (Heard et al, 2004). The site should be cleaned thoroughly before venepuncture takes place.
The lizard should be restrained in dorsal recumbency and the tail supported by an assistant. A towel used to restrain the lizard is advised to protect the handler from injury. The needle is introduced into the proximal third of the midline tail and advanced perpendicular to the tail towards the vertebrae until they come into contact. The needle is retracted slowly while applying negative pressure until blood has been drawn. It must be taken slowly owing to the low volume of blood within this vein (Jenkins, 1996). Larger lizards can be placed in sternal recumbency with the tail placed over the edge of the table and the sample taken from below.
Heart (cardiocentesis)
There is a great level of risk involved with obtaining a blood sample via cardiac puncture. A 23-25 gauge needle is needed and the smallest syringe possible should be used.
The cardiac position in different species can vary. In some, for example, the heart is cranial between the shoulders; in others, the heart is positioned mid-abdomen. It is good practice to use a Doppler flow-detection monitor to locate the exact position of the heart. The cranial heart is approached from the thoracic inlet or from between the ribs. Slight negative pressure should be applied as the needle is directed caudally towards the heart, parallel to the base of the sternum. Often this sample is taken from lizards that are too small to have a sample obtained from the ventral coccygeal vein and are therefore at great risk of cardiac damage. Careful restraint of the lizard is essential and an anaesthetic may be advised for this sample. Sudden movements from the lizard may result in laceration of the cardiac tissue which can result in severe haemorrhage and even death (Jenkins, 1996).
Ventral abdominal vein
The ventral abdominal vein (Figure 6) lies superficially on the ventral midline. A 5/8-inch 25-gauge needle and the smallest syringe possible should be used.

Lacerations of this vein prove a high risk and only a well restrained or anaesthetised patient should have a sample taken from here. The needle should be inserted in the proximal two thirds of the abdominal midline superficially under the skin. This site is not commonly used except in cases where the tail is absent. Particular care should be taken when using this technique.
Squamata — Serpentes
Venepuncture sites commonly used in the Serpente species are:
| Biochemistry | ||
|---|---|---|
| Albumin | g/litre | 18–34 |
| Total protein | g/litre | 46–84 |
| Uric acid | Umol/litre | 77–500 |
| AST | Iu/litre | 9–224 |
| Creatine kinase | Iu/litre | 77–2460 |
| Urea | Mmol/litre | 0–2.14 |
| Sodium | Mmol/litre | 147–183 |
| Calcium | Mmol/litre | 3.00–4.90 |
| Phosphorous | Mmol/litre | 0.61–1.94 |
| Haematology | ||
| White cell count | 10^9/litre | 1.02–12.0 |
| Haemo-globin | g/dl | 9.7–12.0 |
| PCV | % | 21–52 |
| MCHV | g/dl | 32–40 |
| Heterophils | 10^9/litre | 0.135–8.35 |
| Lymphocytes | 10^9/litre | 0.406–22.9 |
| Monocytes | 10^9/litre | 0.042–2.204 |
| Azurophils | 10^9/litre | 0.075–5.338 |
Heart (cardiocentesis)
The heart (Figure 7) lies roughly in the proximal one third of the body; however, this varies depending on the species. A 23-25 gauge needle and the smallest syringe possible should be used.
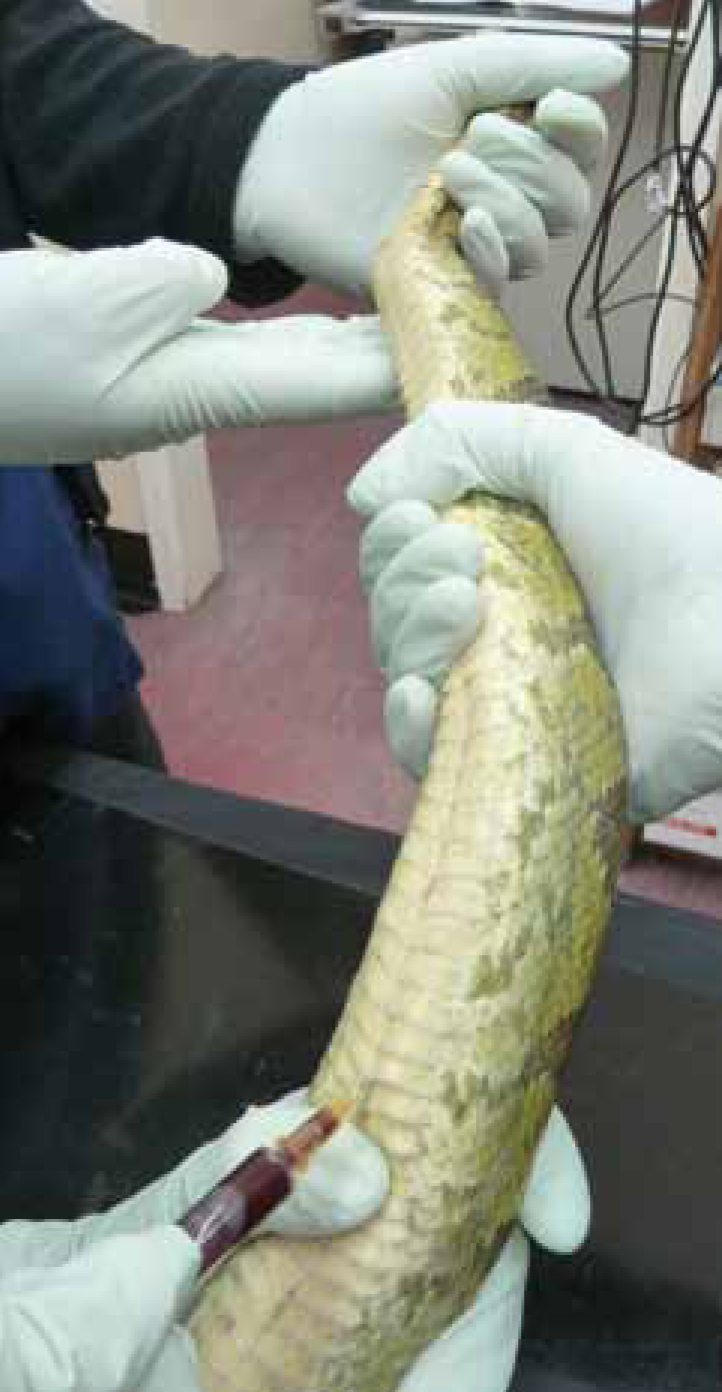
The snake is restrained in dorsal recumbency with gentle pressure placed behind the head, taking care to avoid iatrogenic trauma caused by excessive pressure. The body must be supported and in the case of larger snakes, this may require more than one assistant.
The heart is located by manual palpation, using a Doppler flow-detection monitor or simply by observing the scutes for signs of the heart beating. Once located, the heart should be stabilised with a thumb and finger placed cranially and/or caudally around it (Jenkins, 1996). The needle should be inserted at a 45° angle between the ventral scales and advanced cranially towards the heart. The blood flow will correspond to the movement of the heart. Care should be taken with this sample as there is a high risk of cardiac damage; an anaesthetic may be advised. Sudden movements from the snake may result in laceration of the cardiac tissue which can result in severe haemorrhage and even death.
Ventral coccygeal vein
The ventral coccygeal vein (Figure 8) lies along the ventral midline of the coccygeal vertebrae. A 5/8-inch or 1-inch 23-25 gauge needle (depending on the size of the snake) and the smallest syringe possible should be used. The site should be cleaned appropriately to reduce the risk of faecal contamination.

The snake should be held by an assistant in dorsal recumbency, taking care to stabilise the caudal tail. The needle is introduced into the midline tail, caudal to the vent; it is advanced towards the vertebrae perpendicular to the tail until they come into contact. The needle is retracted slowly while applying negative pressure until blood has been drawn.
Palatine vein
The palatine vein lies on the medial surface of the palatine. The risk of injury from snake bite is great. A 25–27 gauge needle and the smallest syringe possible should be used.
This technique should be used mainly in larger snakes and it is advisable to only obtain this sample under anaesthetic. It is important to prevent contamination from oral fluids. This site is not often used for venepuncture.
Conclusion
Illness and disease are commonly seen in reptiles resulting in the need for further diagnostic steps including venepuncture. There are several venepuncture sites that differ between the Chelonia, Sauria and Serpentes species, and knowledge of these is vital to obtain a diagnostic sample.
Thorough cleaning of the site is important before obtaining a sample and selecting the correct gauge needle and smallest syringe possible is important so as not to lacerate the vein or damage the red blood cells. Care must be taken with the handling of the reptile as autotomy can occur; severe contusions could also result in fatalities. Knowledge of the species and how to correctly handle the reptile is important to obtain a sample of diagnostic quality and to prevent any injury to both the handler and the animal.

