The Kingdom of Tonga’s main island of Tongatapu has a human population of 74611 (Tonga Department of Statistics, 2016), and an estimated canine population of 17 122 dogs, giving a dog:human ration of 1:4 (G Aguilar, unpublished data, 2018), similar to other developing countries such as Nepal, Indonesia and the Philippines (Jackman and Rowan, 2007; Traub et al, 2015). The close physical relationship that dogs have with humans in Tonga, as both pets and guard dogs, inherently increases the risk of disease transmission, since more than 60% of known infectious diseases are zoonotic (Bidaisee and Macpherson, 2014).
This pilot study considers two specific zoonotic pathogens - Leptospira spp. and Dirofilaria immitis - in dogs in the Kingdom of Tonga. Leptospirosis has been identified in 13 Pacific nations, including Tonga (Guernier et al, 2018). However, under-reporting of this disease is common, and it is known to mimic other diseases (Izurieta et al, 2008; Victoriano et al, 2009). Cattle, swine, dogs and rats are common reservoirs for Leptospira spp., and shed leptospires in urine. In countries with tropical climates, such as the Philippines and Thailand, 68-92% of rats were found to carry antibodies for leptospires (Tangkanakul et al, 2005; Villanueva et al, 2010). Dogs infected with Leptospira spp. typically present with fever, jaundice, vomiting, diarrhoea, intravascular disseminated coagulation, renal failure, haemorrhages and death. Infection may occur via direct or indirect exposure, where rivers, soil and water reservoirs may be contaminated by urine of carrier animals (Victoriano et al, 2009; Guernier et al, 2018). Spikes in infection rates in humans are often noted following cyclones with secondary flooding, and projected changes in the world’s climate are expected to increase the frequency of these severe weather events (Gubler et al, 2001; Lau et al, 2016). Clinical signs in humans range from a mild flu-like illness, to severe complications including acute renal failure and pulmonary haemorrhagic syndrome. The latter is associated with high fatality rates (Guernier et al, 2018).
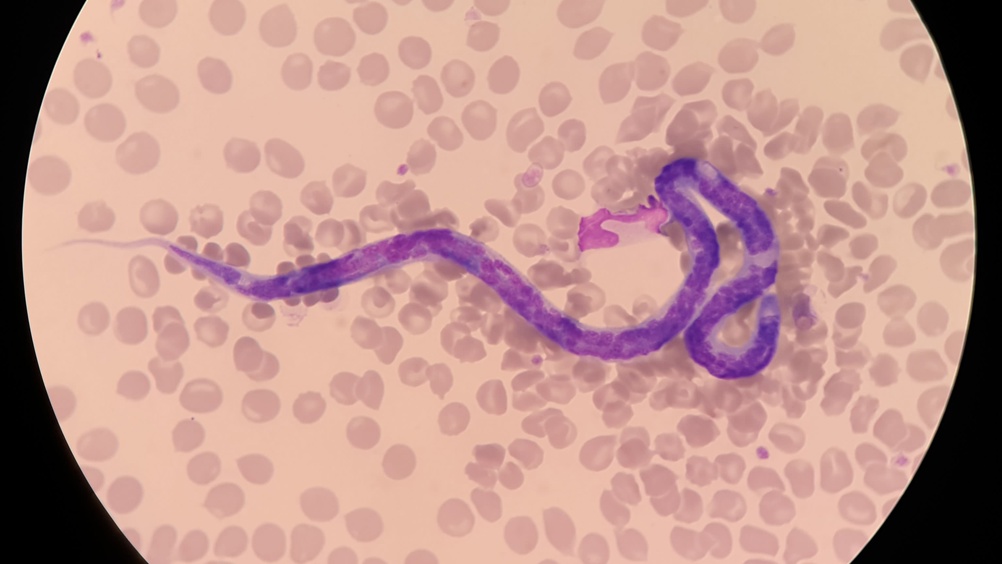
Human infections of D. immitis are unusual, while transmission of this nematode via the mosquito as a vector have been reported (Mendoza-Roldan et al, 2021). It is considered an emerging zoonotic disease that may be transmitted between humans and carnivores in tropical and subtropical regions (Vieira et al, 2014; Anvari et al, 2020). Clinical signs of heartworm caused by D. immitis in dogs include a cough, dyspnoea, congestive heart failure, physical activity intolerance, intravascular haemolysis, haemoptysis, ascites, pulmonary thromboembolism, loss of appetite and weight loss (Anvari et al, 2020). D. immitis may be transmitted to humans in its third larval stage, and transmission may cause benign pulmonary nodules in humans, that can be misdiagnosed as pulmonary carcinomas (Simón et al, 2009; Montoya-Alonso et al, 2011; Vieira et al, 2014).
The zoonotic nature of both pathogens in this pilot study highlight the importance of a One Health approach to disease control, where links between animal, human and environmental health are considered simultaneously.
Leptospira spp. and D. immitis have a higher incidence in tropical countries (Berlioz-Arthaud, 2003; Simón et al, 2009; Montoya-Alonso et al, 2011; Chadsuthi et al, 2017), and dogs living in conditions similar to those found in Tonga (outdoor lifestyle, exposure to raw sewage, scavenging for food, consumption of raw meat, and the prevalence of vectors such as mosquitos), are at a higher risk of exposure to both Leptospira spp. and D. immitis (Meeyam et al, 2006; Carslake et al, 2017). An estimated 10.9% of dogs are infected with D. immitis globally (Anvari et al, 2020).
The lack of Tonga-specific data on these two pathogens is partially because of the limited access to veterinary services, with many Pacific nations, including Tonga, having no permanent veterinary clinic or qualified veterinary personnel. The Kingdom of Tonga relies on volunteer veterinary surgeons and veterinary nurses to provide treatment for animals in the form of temporary clinics. These typically focus on desexing companion animals, with one such clinic used to construct a dataset on the Leptospira spp. and D. immitis prevalence in dogs. While this report is the only current systematic analysis of the presence of these two pathogens in Tonga, it is limited because of sample bias. However, it provides a method to be replicated in future studies of dogs, both in Tonga and other Pacific nations.
Materials and methods
Sample collection
Inclusion criteria included a minimum age of 6 months for dogs and admission to the clinic for desexing. To be eligible for desexing, dogs were required to be clinically healthy. The minimum age was selected because D. immitis is only detectable in infected dogs aged more than 6 months (Santoro et al, 2019). If age was unknown, the dog was aged dentally and any dogs with deciduous dentition were excluded. Of the dogs admitted for desexing, 82 were eligible to be tested for Leptospira spp. antibodies and D. immitis antigens, based on the above criteria.
A blood sample was collected via intravenous catheter during anaesthesia for each dog’s desexing surgery. Blood samples were placed in labelled, additive-free Vacutainer tubes, and stored in a standard refrigerator to allow clotting and separation of blood samples. Gender, age (where known) and weight were recorded. Ethics approval for this study was obtained by the AgResearch Animal Ethics Committee, Ruakura, New Zealand, application number 14611. All experiments were conducted in accordance with AgResearch Animal Ethics Committee guidelines.
Testing
To test for the presence of Leptospira spp. antibodies and D. immitis antigens in situ, canine Leptospira antibody SNAP tests and canine heartworm SNAP tests (IDEXX Laboratories, USA) were used in accordance with manufacturer’s instructions. To obtain serum, 3 ml of serous blood from each dog’s blood sample was extracted via pipette, placed in an Eppendorf tube, and spun at 10 000 RPM in a centrifuge for 3 minutes to separate the serum from red blood cells. Each test required three drops of serum and four drops of conjugate, which were placed in the sample tube provided with each SNAP test. The sample tube was capped and inverted a number of times to thoroughly mix the contents. The entire contents of the sample tube were dropped via pipette into the sample well of D. immitis and Leptospira spp. SNAP tests and labelled with the dog’s identification number. Once the sample had flowed to the activation window, the activator was pressed firmly. Each test was read after the required time, in accordance with package insert instructions. Results were recorded for each dog against their identification number.
Results
A total of 82 dogs, all from Tonga’s main island of Tonga-tapu, met criteria to be tested for both Leptospira spp. and D. immitis. All dogs tested were of mixed-breed, primarily from the Tokomololo village area, and 48% of dogs tested were juvenile (more than 6 months old, but less than 12 months). Ofthese, 39 were male and 43 were female (Table 1). None of the dogs tested had been vaccinated for D. immitis or Leptospira spp.
Table 1. Gender and age distribution of dogs tested for prevalence of Leptospira spp. and D. immitis
| Gender | 6-12months old | 12-24months old | 24+months old | Unknown age | Total |
|---|---|---|---|---|---|
| Male | 15 | 7 | 4 | 13 | 39 |
| Female | 25 | 6 | 7 | 5 | 43 |
| Total | 40 | 13 | 11 | 18 | 82 |
All 82 dogs in this pilot study tested negative for both Leptospira spp. and D. immitis.
Discussion
The lack of D. immitis and Leptospira spp. was unexpected because of prevalence of D. immitis has been reported at 22-86% in Asia Pacific countries (Carslake et al, 2017), and Leptospira spp. has previously been identified in swine and cattle in Tonga (Saville, 1996), with serogroups Australis and Pomona found in both species, Hebdomadis, Sejroe and Tarassovi found only in cattle, and Icterohaemorrha-giae found only in swine. The serovars Canicola, Icterohe-morrhagiae, Grippotyphosa, and Pomona are commonly found in dogs (Izurieta et al, 2008). No previous quantitative data for D. immitis in Tonga has been identified, but nearly 47% of dogs studied in the neighbouring Pacific nation of Samoa were found to carry D. immitis antigens (Carslake et al, 2017).
This pilot study was limited to one species (dogs). Future studies into the presence of Leptospira spp. in particular may be expanded to include additional species, such as rats, swine and cattle, as well as the testing of water samples, to further investigate the presence of leptospires. Polymerase chain reaction testing has been successfully used to detect leptospires in water, to test for the presence of the lipL32 protein (Munoz-Zanzi et al, 2014; Wynwood et al, 2014).
This study was also limited by sample bias and the testing method available. Based on these limitations, and because of the limited data available on the health status of animals in Tonga, further development and expansion of this pilot study is warranted.
Sample population
For both tests, dogs were not randomly selected to be tested at the clinic, thus introducing bias in the sample population. Further studies should include other regions of Tongatapu, as well as other islands of Tonga where possible, to provide access to a geographically wider sample population. Future studies should include dogs that may not ordinarily be presented at a veterinary clinic, including those who are not clinically healthy.
The health of dogs in Tonga is challenged by poor nutrition and limited access to preventative health care (K Harder et al, unpublished data, 2023), meaning that a dog may not survive when challenged by a pathogen such as Leptospira spp. or D. immitis. Infection and subsequent development of leptospirosis can carry high mortality rates of up to 43.4% (Major et al, 2014), with younger dogs being more susceptible to peracute or acute death (Rissi and Brown, 2014). The sample for this study was selected from dogs presenting for desexing at a temporary spay/neuter clinic set up for 1 week. However, to be eligible for desexing, all dogs underwent a clinical exam to confirm they were clinically healthy. Many dogs in Tonga are not desexed, resulting from the lack of access to veterinary treatment and, anecdotally, negative owner perceptions around the need for desexing. This puts entire male dogs, in particular, at higher risk for infection with Leptospira spp. because of their greater roaming and contact with urine (Major et al, 2014).
Testing method
Three methods are commonly used to identify the presence of Leptospira spp.: enzyme-linked immunosorbent assay, microscopic agglutination test and polymerase chain reaction test. The latter two require specialised testing equipment and personnel, not available in Tonga. Therefore, microscopic agglutination test and polymerase chain reaction test would require transporting biological samples to a location with suitable facilities and technicians, imposing environmental and border security risks. In addition, microscopic agglutination test testing has been reported as subjective for leptospirosis (Winzelberg et al, 2015), with discordant results shown from various testing laboratories (Miller et al, 2011). The chosen method of enzyme-linked immunosorbent assay using SNAP tests (IDEXX), is commonly used in veterinary clinics as a ‘point-of-care’ test. Curtis et al (2015) identified a 79.2% overall agreement of SNAP tests with microscopic agglutination testing, with variations based on titre levels. However, alternative enzyme-linked immunosorbent assay tests, in the form of the WITNESS Lepto Rapid Test (Zoetis, USA), have shown improved performance when compared with SNAP tests and microscopic agglutination test (Lizer et al, 2017).
‘The health of dogs in Tonga is challenged by poor nutrition and limited access to preventative health care’
The most common serovars of L. interrogans thought to infect dogs, before the introduction of vaccination in the 1980s, were Icterohaemorrhagiae and Canicola (Sykes et al, 2011), and the L. interrogans serovar Pomona strain has been previously identified in cattle and swine in Tonga (Saville, 1996). The serogroups Icterohaemorrhagiae and Australis, commonly found in rats, were identified in the western Pacific islands (Victoriano et al, 2009). Serovars in the SNAP tests used are limited to Grippotyphosa, Canicola, Pomona and Icterohaemorrhagiae. However, additional serovars not included in tests used in this pilot study, may be present in Tonga.
While no positive tests were returned in this pilot study, Leptospira spp. has previously been detected in livestock in Tonga, and the practice of keeping dogs as pets is becoming more common in developing countries, possibly as a result of increasing urbanisation and wealth (Traub et al, 2015). This increase in both population and interaction between dogs and humans brings possible additional opportunities for disease transmission, with free-roaming dogs more likely to transmit disease (Farnworth et al, 2012).
Conclusions
While this pilot study found no evidence of Leptospira spp. or D. immitis in the sample of dogs, further studies are warranted, in particular for Leptospira spp. because of previous evidence found in Tonga and the global importance of leptospirosis. Expanding sampling options may assist in developing a clearer idea of the presence of these pathogens in Tonga. Environmental sampling of water for evidence of leptospires may identify non-mammalian reservoirs, and sampling of additional hosts such as cattle, swine and rats may identify mammalian reservoirs.
Key Points
- Dirofilaria immitis and Leptospira spp. have been identified in Tonga, but no previous studies have been carried out on their presence in dogs.
- Both infectious diseases are zoonotic, which poses a public health risk.
- This pilot study aimed to develop a methodology to identify the presence of both diseases in dogs in Tonga. Future studies are warranted, and a refined method is suggested.