This article evaluates the current literature sur-rounding the veterinary nurse's role in the post-operative care of the brachycephalic patient – specifically airway management and cardiovascular support.
In recent years, there has been a dramatic increase in ownership of brachycephalic breeds (British Veterinary Association, 2018). The exaggerated morphological features and related health concerns, alongside a higher incidence of inherited disorders, has contributed to an ever-increasing caseload of brachycephalic patients in practice (O'Neill et al, 2022). Surgical interventions are commonly required in the treatment of disorders such as brachycephalic obstructive airway syndrome (BOAS), dystocia, dental disease and intervertebral disc disease (Gyles, 2017).
It is well-documented in the literature that complications following surgery in the brachycephalic patient are most likely to occur in the postoperative period (Grubb, 2022) – with higher rates of mortality associated with the performance of airway surgery (Downing and Gibson, 2018). As death is most likely to occur postoperatively within the initial 3 hours of recovery, close monitoring of the patient during this time may reduce mortality rates, while also improving nursing care provision (Crompton and Hill, 2011).
A review of the literature relating to the postoperative care provided to brachycephalic animals has identified common postoperative complications including dyspnoea, aspiration pneumonia, regurgitation, hypoxia, hypothermia, hyperthermia, bradycardia, arrhythmias and respiratory acidosis. The high resting vagal tone of the brachycephalic animal can easily be stimulated and induce bradycardia. As such, careful movement of the patient is essential.
This article reviews the provision of nursing care and nursing interventions in the initial postoperative period, focusing on airway management and cardiovascular support. Nursing practice recommendations for the postoperative brachycephalic patient are included. Long-term hospitalisation considerations, laboratory tests and surgical success rates are not reviewed here.
Airway management
Brachycephalic obstructive airway syndrome
BOAS is a is a lifelong, debilitating obstructive airway disease that has a significant impact upon the quality of life of the breeds of dog affected (Mitze et al, 2022). Such breeds include the French Bulldog, Pug and Boston Terrier (Ladlow et al, 2018). In the brachycephalic animal, the primary anatomical abnormalities associated with BOAS include stenotic nares and elongated soft palate, which obstruct the upper airway – reducing air flow to the trachea and sub-sequently increasing respiratory effort and negative airway pressure. In such cases, secondary inflammation may occur – alongside soft tissue changes such as laryngeal oedema and collapse (Adshead, 2014). The clinical signs of BOAS are highlighted in Table 1.
Table 1. Clinical signs of brachycephalic obstructive airway syndrome
| Stridor | Exercise intolerance | Syncope |
| Stertor | Cyanosis | Abducted elbows/wide stance |
| Dyspnoea | Hyperthermia | Sleep apnoea |
Intubation
Maintaining the airway via intubation for the recovering patient is vital to maximise airflow and reduce respiratory effort. Poor ventilation is associated with the development of hypoxia and negative-pressure pulmonary oedema (Gruenheid et al, 2018). The endotracheal tube should remain in place until no longer tolerated (Scales and Clancy, 2020). The endotracheal tube should be removed if the patient becomes agitated, distressed or starts trying to chew the tube. Extubation should be performed slowly, as the upper airway may be oedematous or inflamed (Grubb, 2022). Negative airway pressure and an increased incidence of gastrointestinal malformation, such as hiatal hernia, predisposes brachycephalic breeds to the development of gastro-oesophageal reflux and regurgitation (Appelgrein et al, 2022). A study by Fenner et al (2020) involving 258 dogs found younger dogs to be at higher risk of postoperative regurgitation – associated with an immature oesophageal sphincter. Furthermore, dogs with a previous history of regurgitation were identified as at higher risk.
Regurgitation and aspiration pneumonia
Recovering the brachycephalic patient in sternal recumbency, with the head and neck elevated, reduces the risk of the aspiration of regurgitant fluid (Adshead, 2014). Partial deflation of the endotracheal tube cuff before extubation allows potential regurgitant fluid to be pulled from the airway. However, delayed extubation can itself trigger regurgitation (Scales and Clancy, 2020). Aspiration pneumonia may develop following the aspiration of contaminated material in to the larynx and lower respiratory tract. The development of acute respiratory distress syndrome resulting from aspiration pneumonia was found to be associated with increased mortality (Balakrishnam et al, 2017).
A study focused on the risk of anaesthesia-related post-operative complications in brachycephalic dogs by Gruenheid et al (2018) identified aspiration pneumonia to be the most common postoperative complication – with 9 out of 39 dogs studied developing the condition. As increased negative intrathoracic pressure increases the risk of gastro-intestinal dysfunction (Lindsay et al, 2020), the incidence of aspiration pneumonia postoperatively is linked with the severity of BOAS that the patient presents with (Gruenheid et al, 2018).
Pre-emptive measures to reduce the incidence of gastro-oesophageal reflux and regurgitation are essential – reducing the risk of subsequent aspiration pneumonia. The administration of gastroprotectants, anti-nausea medications and prokinetics may commence in the peri-anaesthetic period and be continued postoperatively (Gruenheid et al, 2018). Alongside reflux and regurgitation, the upper airway is at risk of obstruction in the postoperative period as a result of mucosal oedema, active bleeding or blood clots (Downing and Gibson, 2018). Inspection of the airway and clearance of accumulated debris before extubation – using suction or gauze swabs – is advisable (Adshead, 2014). While the patient undergoing sedation or general anaesthesia will usually undergo a period of pre-operative fasting, prolonged fasting increases the risk of gastro-oesophageal reflux in the brachycephalic patient. The presence of a hiatal hernia is also a risk factor for gastro-oesophageal reflux (Grubb, 2022).
Ventilation
Following extubation, oxygen saturation should be monitored, with equipment ready to re-intubate the patient if indicated (Nutbrown-Hughes, 2020). Pulse oximetry (SpO2) readings of less than 94% oxygen saturation indicate the need for supplemental oxygen (Miller and Gannon, 2015). However, pre-operative readings of 95% are commonplace in the BOAS patient on room air (Woodlands, 2018), because of their lower partial pressure of oxygen (Scales and Clancy, 2020). As such, following extubation, oxygen saturation for the brachycephalic breathing room air may not exceed 95% SpO2. A pre-operative reading of SpO2 may help to guide the veterinary nurse's expectations postoperatively (Scales and Clancy, 2020).
The adequacy of ventilation is ideally assessed using arterial blood gas analysis – with hypercapnia indicative of hypoventilation and a decrease in pH, increase in PCO2 and increase in HCO3 indicative of respiratory acidosis (Sidari, 2021). Normal blood gas values and ranges are shown in Table 2 (Sidari, 2021).
Table 2. Normal blood gas values and ranges
| Canine | Arterial | Venous |
|---|---|---|
| pH | 7.351–7.463 | 7.351–7.443 |
| PO2 (mmHg) | 80.9–103.3 | 47.9–56.3 |
| PCO2 (mmHg) | 30.8–42.8 | 33.6–41.2 |
| HCO3 (mEq/litre) | 18.8–25.6 | 20.8–24.2 |
| Feline | ||
| pH | 7.31–7.462 | 7.277–7.409 |
| PO2 (mmHg) | 95.4–118.2 | 112.0 |
| PCO2 (mmHg) | 25.2–36.8 | 32.7–44.7 |
| HCO3 (mEq/litre) | 14.4–21.6 | 18.0–23.2 |
Respiratory acidosis is a common finding in the brachycephalic animal, as a result of pre-existing respiratory depression, low tidal volume and fast respiratory rate (Fawcett et al, 2018). However, respiratory acidosis may occur in any patient that is hypoventilating and unable to eliminate carbon dioxide effectively – with causes of hypoventilation including neuromuscular disease, pleural disease and drug-induced depression of the respiratory centre (Poli, 2022). A low tidal volume directly limits alveolar gas, resulting in acidosis (Fawcett et al, 2018). Capnography is another useful tool in monitoring the efficiency of ventilation (Prisk, 2019). Side-stream capnography can provide insights into air flow through the stenotic nares and an end tidal carbon dioxide (ETCO2) measurement. However, as with other forms of monitoring, results should be considered as a trend, as readings may be inaccurate as a result of dilution from room air (Scales and Clancy, 2020). Normal ETCO2 readings in the non-anaesthetised animal are 35–45 mmHg – with values above 50 mmHg indicating inadequate ventilation (O'Dwyer, 2015). However, BOAS patients have an increased partial pressure of carbon dioxide (PaCO2) compared with meso- or dolichocephalic breeds, because of their existing upper airway disease (Adshead, 2014). While the provision of manual or mechanical ventilation should be considered on an individual basis considering clinical signs, ETCO2 should not exceed 55 mmHg (Adshead, 2014).
In the postoperative period, drug-induced respiratory depression and hypothermia increase the work of breathing in the BOAS patient – altering air flow and increasing the risk of secondary pulmonary oedema (Downing and Gibson, 2018). Oxygen debt may occur with hypothermia, as shivering increases oxygen consumption (Grubb, 2022). Hypothermia is also associated with coagulation deficiencies, impairment of platelet function and prolonged recovery times (Scales and Clancy, 2020).
Oxygen supplementation
A study by Lindsay et al (2020), following the surgical cor-rection of BOAS, found that dyspnoea on initial recovery may be associated with haemorrhage, anxiety or pharyngeal swelling. However, as the study data included patients presenting with respiratory distress and those admitted for elective surgery, it is likely that some degree of pharyngeal swelling and anxiety was also present pre-operatively. In 58 of the 248 dogs studied, the complications described above were observed. However, 18 dogs were treated with supplemental oxygen alone for dyspnoea and required no further intervention – with nasal oxygen catheters used in each case. Oxygen supplementation in the immediate postoperative period is highly beneficial – with a tracheal catheter effective at increasing the fraction of inspired oxygen and arterial oxygen partial pressure (Packer and Tivers, 2015). A nasotracheal tube is an alternative to a percutaneous tracheal catheter, but both require placement under general anaesthesia. While intubated, oxygen may be supplemented with a catheter into the endotracheal tube (Lindsay et al, 2020). The demeanour and size of the patient are factors when considering postoperative oxygen supplementation – with larger dogs more tolerant of nasal lines, and some not able to tolerate a face-mask or flow-by provision. The use of an oxygen-rich environment may be considered but caution should be exercised – with the brachycephalic patient particularly susceptible to overheating (Prisk, 2019).
As highlighted by Lynch (2018), long-term exposure to oxygen supplementation may promote the drying of the respiratory epithelium and the subsequent development of respiratory infection and, as such, humidification through pulling oxygen through sterile saline or water is recommended.
Body temperature
The optimum temperature for the recovering brachycephalic patient is recognised as 36°C – with active warming used until this temperature is reached (Scales and Clancy, 2020). While still hypothermic, it is not recommended to raise body temperature past this marker – as excessive panting may be seen in the normothermic patient (Scales and Clancy, 2020). Hyperthermia increases respiratory drive, with excessive negative pressure required to move larger volumes of air – as such, swelling of the upper airway ensues and with it, compromised airflow and heat dissipation (Fawcett et al, 2018). A cycle of heat stress and upper airway obstruction then follows (Fawcett et al, 2018). Furthermore, prolonged hyperthermia/heat stress may cause organ injury – with the gastrointestinal tract most susceptible to damage. Active cooling via intravenous fluid therapy, a fan in front of patient and wetting the fur with room temperature water is performed when body temperature exceeds 39.5°C. However, to prevent rebound hypothermia, active cooling past 39.4°C is not recommended (Fawcett et al, 2018).
Monitoring
Owing to the risk of postoperative respiratory compromise, all equipment to facilitate re-intubation of the patient, perform a tracheotomy and induce anaesthesia should be prepared well in advance of recovering the patient (Devonshire, 2016). Assigning a dedicated nurse for the recovery period to provide continuous monitoring is preferable (Devonshire, 2016). A study by Costa et al (2020) found that recovering the brachycephalic patient in an intensive care unit (ICU) enabled a higher standard of monitoring, resulting in early identification of complications and immediate intervention. However, a dedicated ICU may not be widely available in general practice. When monitoring the recovering patient, observing a pattern in vital signs rather than a one-off ‘single’ measurement provides more relevant information (Jones, 2022). As discussed individually above, respiration monitoring in the postoperative period should include: pulse oximetry, capnography, blood gas analysis and body temperature. Additionally, the observation of chest movements, respiration rate and character – alongside regular thoracic auscultation, throughout recovery and hospitalisation – to identify respiratory impairment is essential (Gurney, 2011).
Cardiovascular support
An assessment of cardiovascular function in the patient can easily be performed using subjective methods such as mucous membrane colour, capillary refill time, pulse rate and quality, peripheral and core body temperature (Homer, 2020). As basic markers of perfusion, abnormalities in findings may lead to the use of advanced monitoring tools such as electrocardiography (ECG), blood pressure measurement and pulse oximetry). A perfusion deficit results in reduced perfusion of tissues and subsequent reduction in oxygen delivery – resulting in organ dysfunction and eventual organ failure (Poli, 2021).
Electrocardiogram
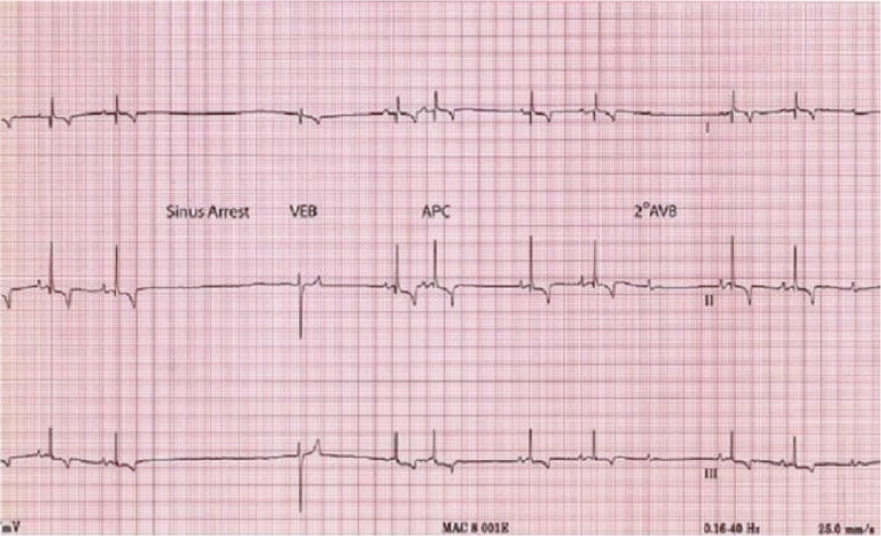
Brachycephalic breeds have a high resting vagal tone – which may commonly result in bradycardia (Prisk, 2019). Vagal tone may also be referred to a parasympathetic tone – a subsection of the autonomic nervous system, which governs the motor function to smooth and cardiac muscle (Rausch, 2008). The autonomic nervous system has a central regulatory function, which maintains homeostasis through multiple through coordinated influences on multiple organ systems (McLaughlin et al, 2015). Named vagal after the largest cranial nerve (X) of the parasympathetic nervous system (Laborde et al, 2017), vagal tone is a measure of parasympathetic control over heart rate (McLaughlin et al, 2015). Stimulation of vagal tone may occur during intubation, extubation and movement of the patient (eg sternal recumbency to lateral recumbency) (Scales and Clancy, 2019a; b). Careful positioning and avoiding overstimulation during intubation and extubation is essential (O'Dwer, 2017). Sinus arrest may be identified via electrocardiogram (associated with vagal tone during inspiration) and is identified as a pause between complexes, that is greater than two times the normal R to R interval (O'Dwyer, 2019) (Figure 1). Second-degree atrial-ventricular (AV) block and syncope are also common findings associated with high resting vagal tone (Scales and Clancy, 2020).
Blood pressure
Hypertension has been reported to be higher in incidence in the brachycephalic animal in comparison to mesocephalic and dolichocephalic breeds (Lindsay et al, 2020). Pulmonary hypertension may occur in the brachycephalic patient due to chronic hypoxia and resulting pulmonary vasoconstriction (Scales and Clancy, 2020). The monitoring of blood pressure in the recovering patient may be achieved by indirect (Doppler/oscillometric) or direct (arterial) methods (Forsyth, 2007).
Considered the gold-standard technique, providing systolic, diastolic and mean arterial blood pressure measurements, direct/invasive blood pressure measurement requires the placement of an arterial catheter; posing a greater risk relating to haemorrhage should the catheter become dislodged (Jones, 2022). However, as the brachycephalic patient is often continuously monitored in the recovery period, the risk of dislodgement may be reduced. As a result of the costs involved and the specialist equipment required for arterial blood pressure measurements, this technique is often used for the intensive care patient (Jones, 2022).
While indirect methods of blood pressure measurement such as Doppler and oscillometry are often readily available in practice, their use in the brachycephalic animal may prove difficult. Poor conformation of the forelimbs may hinder the placement of an appropriately-sized blood pressure cuff (40% of limb circumference) – resulting in an overly loose cuff and inaccurate readings (Scales and Clancy, 2020). As such, placement of the cuff below the hock is considered preferable (Scales and Clancy, 2020). However, this was not evidenced through data collection and requires further research. The use of hind limbs for indirect blood pressure measurement is supported by Clapham (2011), with the cuff placed proximal to the hock. The tail base is also proposed as a suitable cuff site but neither recommendation was supported by data analysis. However, as brachycephalic patients often have a screw-tail, this method may not be advisable. Generally, blood pressure readings obtained by indirect methods are considered less accurate than direct, but all readings should be considered as a trend, rather than stand-alone measurement (Clapham, 2011).
Additional cardiovascular monitoring techniques
Pulse oximetry is a useful technique to monitor postoperative pulse rate. As discussed previously, the measurement of arterial oxygen saturation of haemoglobin is also useful in the assessment of ventilation. The pulse rate displayed on the oximeter monitor should be compared to that of a peripheral pulse – with the pulse quality noted. The pulse rate must be within 5 beats per minute of the peripheral pulse to provide an adequate measurement of oxygen saturation (Gurney, 2011). A peripheral pulse assessment (eg dorsal pedal) is preferable a central pulse (femoral), as these are the first to alter with changes in perfusion and circulation (Crompton and Hill, 2011).
Central venous pressure is considered a useful tool for monitoring the effect of fluid therapy in the critical care patient – used to detect hypervolaemia and hypovolaemia (O'Dwyer, 2011). As it requires placement of a central catheter, the use of central venous pressure measurement is contraindicated in cases at risk of coagulopathies (O'Dwyer, 2011). Heat stress is commonly associated with coagulopathies in brachycephalic animals and, as such, the measurement of central venous pressure may not be recommended and certainly requires further research (Fawcett et al, 2018).
Conclusions
The brachycephalic patient presents some unique challenges during the postoperative monitoring period. The anatomical abnormalities often seen with BOAS increase the incidence of postoperative complications such as aspiration pneumonia, regurgitation, dyspnoea and heat stress. Conformational abnormalities of the brachycephalic hinder the placement and use of monitoring tools in the postoperative period, which can alter the accuracy of readings/measurements taken – impacting care provision and potentially treatment outcomes.
The veterinary nurse must be aware of the specific considerations for monitoring of the brachycephalic patient and be fully prepared before receiving the patient to recover from anaesthesia. Maintaining an airway and assessing the adequacy of ventilation throughout recovery is essential to reduce the incidence of complications. Oxygen supplementation is often indicated for the brachycephalic, with the technique used often selected as that best tolerated by the patient.
Cardiovascular monitoring can range from basic techniques such as pulse rate and quality, to advanced methods such as ECG, central venous pressure and arterial (invasive) blood pressure. An understanding of common changes seen in parameters due to brachycephalism is essential including bradycardia, arrythmias and hypertension, alongside high-resting vagal tone.
Reviewing literature in support of this report has high-lighted the lack of veterinary studies dedicated to postoperative monitoring in the brachycephalic patient and further research in this field is essential to encourage the provision of advanced nursing care to these high-risk patients. With the ownership of brachycephalic animals increasing nationwide, we must continue to improve our understanding of their complex requirements.
KEY POINTS
- Intubation must be maintained for as long as is tolerated by the patient – to protect the airway and ensure adequate ventilation, while also enabling the direct provision of oxygen supplementation.
- Postoperative pulse oximetry enables the assessment of oxygen saturation of haemoglobin, with a measurement of 95% commonplace in the brachycephalic animal breathing room air alone – commonly occurring as a result of brachycephalic obstructive airway syndrome.
- Monitoring basic cardiovascular markers such as mucous membranes, capillary refill time, pulse rate and pulse quality may indicate perfusion deficits and advanced monitoring techniques such as electrocardiography (ECG) and blood pressure measurement is advised.
- ECG abnormalities can be associated with high-resting vagal tone and may include sinus arrest and second-degree atrial-ventricular block.
- Chronic hypoxia and pulmonary vasoconstriction predispose the brachycephalic animal to hypertension.


