The aim of this article is to highlight the main complications when anaesthetising dogs with brachycephalic obstructive airway syndrome (BOAS) and to provide clinical techniques to reduce instances of complications.
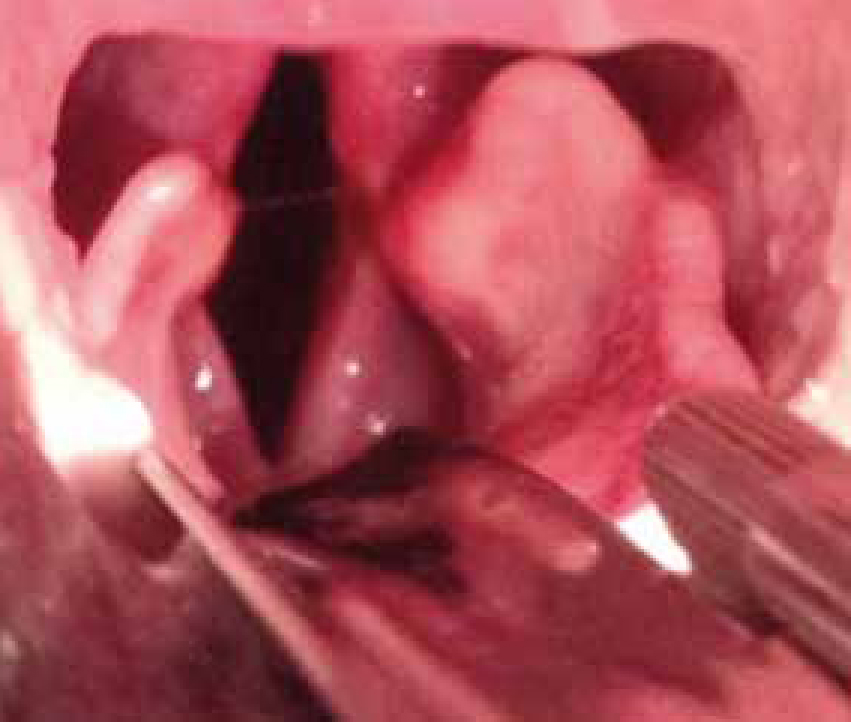
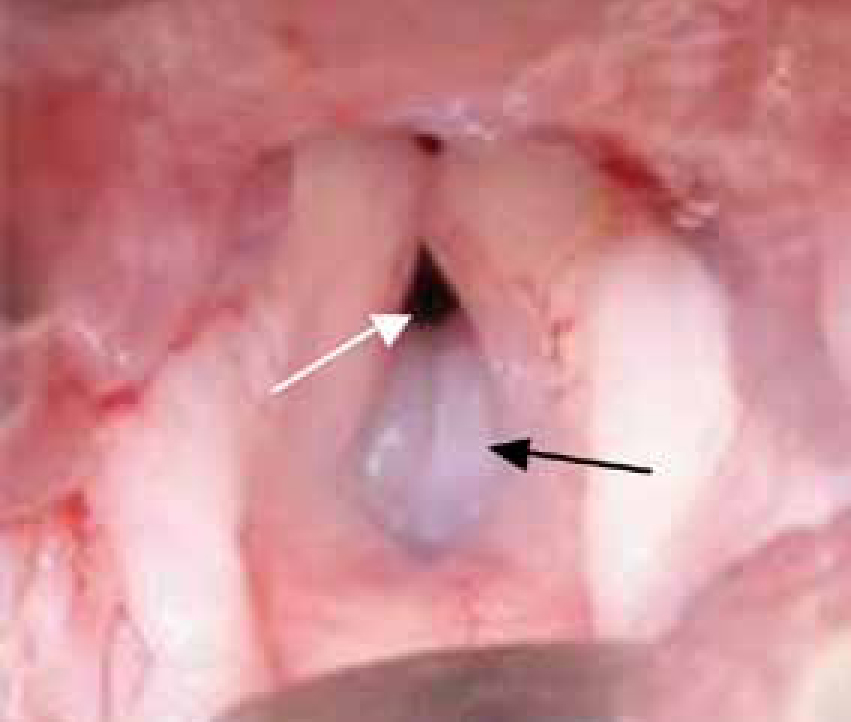
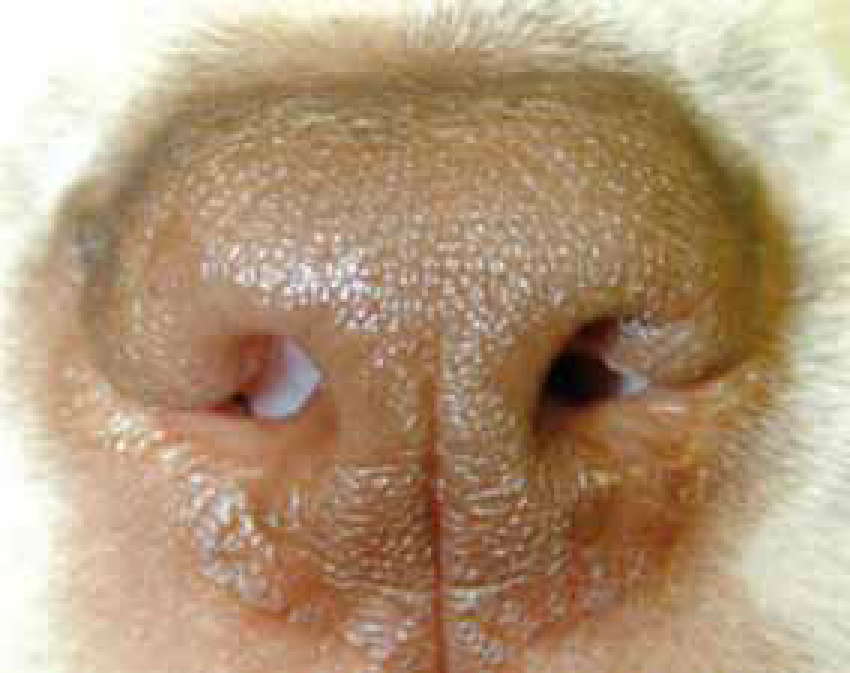
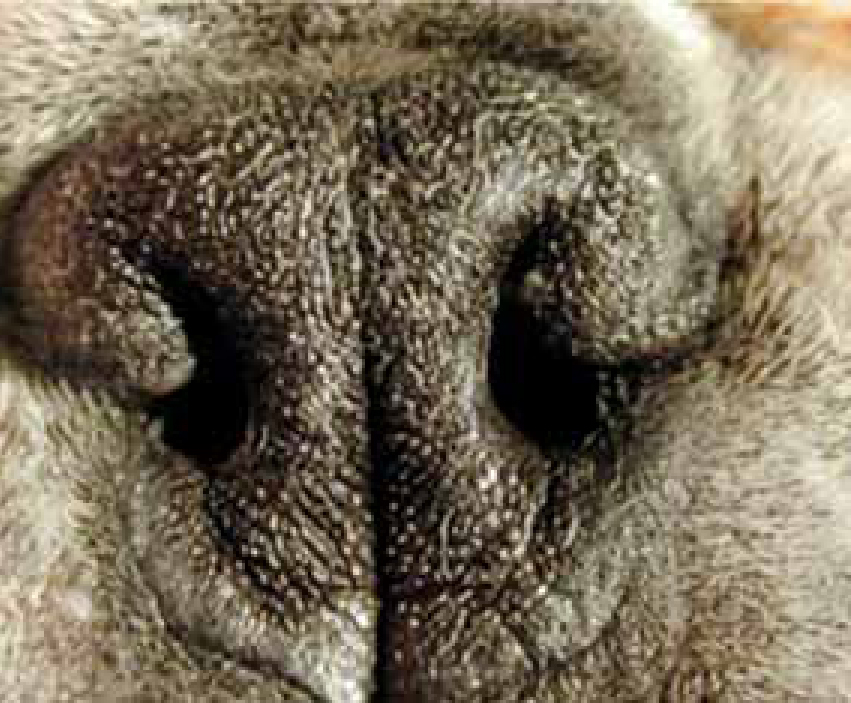
BOAS is a combination of abnormalities of the upper respiratory tract in dogs that can result in obstruction of the upper airway and dyspnoea. Abnormalities include stenotic nares, elongated soft palate, hyperplastic tonsils, everted laryngeal saccules, laryngeal collapse and hypoplastic tracheae. In addition, some patients can have a concurrent sliding hiatal hernia. Affected dogs may have a combination of these disorders, resulting in varying degrees of upper airway obstruction (Figure 1).
Commonly affected breeds include Pugs, Boston Terriers, English and French Bulldogs, Pekingese, Shih Tzus, Boxers, Bull Mastiffs and Cavalier King Charles Spaniels.
Clinical signs include, inspiratory stertor, stridor, exercise intolerance, gagging, regurgitation, vomiting, syncope, and dyspnoea. Clinical signs occur at varying degrees of severity, from mild nasal airflow obstruction to severe airway occlusion. Patients can also suddenly deteriorate when in a stressful situation such as exercise and excitement and may require emergency treatment for severe dyspnoea and cyanosis. The hospital environment can be stressful and care should be taken to make it as quiet and stress free as possible for patients with BOAS.
Physiology
Primary abnormalities include stenotic nares and elongated soft palate, which result in obstruction of the upper airway and reduced flow of air into the trachea. Reduced airflow leads to increased respiratory effort and chronic increased negative airway pressure. A comparative example would be a human breathing through a straw; much more respiratory effort is required to inhale and exhale each breath than in a patient without abnormalities. Eventually, the increased respiratory effort and negative airway pressure can cause secondary inflammatory and soft tissue changes (Torrez and Hunt, 2006), for example laryngeal saccule eversion, laryngeal oedema and, in severe cases, laryngeal collapse.
Anaesthetic complications
Anaesthetic complications associated with BOAS are variable depending on the extent of disease and each patient should be assessed individually. Patients with minimal disease can be stable during the anaesthetic period, while patients with marked clinical signs, such as pronounced airway noise, reduced exercise tolerance, regurgitation, cyanosis and collapse are at high risk of complications, for example, upper airway obstruction and hypoxia.
Intravenous access
Good intravenous (IV) access is important and all BOAS patients undergoing anaesthesia should have an IV catheter. Never administer drugs ‘off the needle’, including anaesthetic induction agents, for example propofol, due to risks of peri-vascular injection and the possibility of a partially anaesthetised patient with an occluded upper airway. An IV catheter will also provide immediate access for emergency drug administration.
Some patients resist IV catheter placement, which can be frustrating and the resistance offered can place the patient at high risk of upper airway obstruction, especially when additional restraint is used. Techniques to aid IV catheter placement include EMLA cream, a topical local anaesthetic that produces cutaneous analgesia. From the author's clinical experience, EMLA does appear to be effective in reducing pain associated with IV catheterisation. It has been shown to be an effective local anaesthetic during cephalic venepuncture in dogs, cats and rabbits (Flecknell et al, 1990) and jugular venepuncture in cats (Wagner et al, 2006), and in reducing pain in human patients (Rogers et al 2004).
An alternative to EMLA is IV catheterisation using the lateral saphenous vein. From clinical experience, the lateral saphenous vein is better tolerated by BOAS patients compared to cephalic catheterisation, perhaps because saphenous catheterisation is less intimidating. Once anaesthetised, saphenous catheters can be exchanged for cephalic catheters if cephalic catheters are preferred.
Intramuscular (IM) sedation should be considered in patients that continue to resist IV catheter placement, especially if additional restraint is causing unnecessary stress. Sedative drugs are discussed in the premedication section of this article.
Sedation and premedication
Sedation or premedication should be considered for patients that are anxious and for patients scheduled for anaesthesia. Drugs used during sedation and premedication can relax naso- and oropharyngeal smooth muscle (Clutton, 2007), which may exacerbate upper airway obstruction. Sedative effects are dose-dependent and higher doses may result in excessive and unwanted sedation. Once sedated, continual airway observation should be provided.
Benefits of sedation and premedication
The benefits of sedation and premedication include:
Many BOAS dogs respond well to sedative-anxiolytic drugs, for example acepromazine (ACP) in conjunction with an opioid; however sedative dosages should be less than that used for non-brachycephalic dogs due to excessive sedation leading to loss of upper airway control (author's experience). IV administration of sedative drugs can be beneficial because the drugs can be administered slowly to effect rather than a standard IM dose, which could cause excessive sedation. See Table 1 for sedation protocols.
| Acepromazine (ACP) 0.005–0.02 mg/kg intramuscular (IM) or intravenous (IV) |
| Good sedative effects |
| Can be used alone or in conjunction with opioids |
| Improved sedative effects when used in combination with an opioid |
| IM or IV admin |
| No analgesic effects |
| Lower doses recommended depending on severity of disease (0.005 mg/kg IM or IV) |
| Excitable dogs may require 0.01–0.02 mg/kg IM in combination with an opioid |
| Onset of action 30–45 mins IM |
| Duration of action — dose dependent (approx 6 hours) |
| Butorphanol 0.1–0.2 mg/kg slow IV or IM |
| Good sedative effects when used alone or in conjunction with ACP |
| Improved sedative effects IV |
| Rapid onset (10–15 mins) |
| Short acting (60–90 mins) |
| Short acting effects are advantageous if adverse effects noted |
| Poor analgesic, not recommended in surgical patients |
| Methadone 0.1–0.2 mg/kg IM |
| Good analgesic effects |
| Can be used alone or in conjunction with ACP |
| Can be administered IV but may result in bradycardia and high doses may cause respiratory depression |
| Slow onset of action — 30 mins |
| Analgesic effects last 4 hours approx |
| Buprenorphine 0.01–0.02 mg/kg IM or IV |
| Mild sedative effects |
| Can be used alongside ACP |
| Moderate analgesic effects |
| Peak effect — 45 mins |
| Duration of action — 6–8 hours |
| Pethidine 1–2 mg/kg IM ONLY |
| Risk of histamine release if given IV — contraindicated |
| Mild sedative effect |
| Mild/moderate analgesic |
| Rapid onset 10–15min |
| Short acting — approximately 1 hour |
| Short acting effects are advantageous if adverse effects noted |
Premedication and sedative drugs have been associated with decreased tear production and subsequently corneal drying can be observed (Thomson, 2007). Additional eye lubrication should be considered. Application of eye lubrication should commence following premedication and should continue post operatively for 12–24 hours as a reduction in tear production can continue for 24 hours post anaesthesia (Thomson, 2007).
Additional IM sedation should be considered in patients that continue to resist IV catheter placement after ACP/opioid premedication. Medetomidine administered IM can offer a safer alternative to additional restraint (personal experience). Patients receiving medetomidine should be monitored continuously due to increased sedative effects and the risk of airway obstruction. An IV catheter should be placed once the patient is sedated and an endotracheal (ET) tube, induction agent, laryngoscope and supplemental oxygen should be ready in cases of airway difficulty.
Anaesthesia induction
Masked or chamber induction of anaesthesia is not recommended due to stress and delayed airway control. It also places susceptible patients at high risk of regurgitation and aspiration (Panti et al, 2009).
Anaesthetic induction is best achieved using IV induction agents, for example propofol and alfaxalone (Alfaxan, Jurox, UK) as IV induction allows for rapid ET tube intubation and airway control. An ideal induction agent does not exist and all induction agents have advantages and disadvantages — using a familiar drug may reduce the chance of human error. The safety of the patient relies on the appropriate and correct handling of the patient at every stage of the procedure.
Advantages and disadvantages of propofol and alfaxalone
Propofol and alfaxalone are routinely used successfully as IV induction agents in BOAS patients. They both provide a smooth induction of anaesthesia and rapid ET tube intubation in pre-medicated patients. They are both short acting and allow for a quick and complete recovery from anaesthesia, with minimal residual effects.
Post-induction respiratory depression and apnoea are potential risks associated with propofol and alfaxalone if administered rapidly during the induction of anaesthesia (Amengual et al, 2013). In susceptible patients, post-induction apnoea can lead to sudden de-saturation and cyanosis, which can be disastrous in a patient with an occluded upper airway, at risk of a difficult intubation. In a clinical setting, alfaxalone appears to cause less post-induction apnoea compared with propofol. Post-induction apnoea can be minimised when propofol and alfaxalone are used simply by slow administration (Table 2).
| Propofol |
| Always pre oxygenate |
| Consider premedication — 20–80% reduction in propofol dose in pre-medicated patients |
| Premedication reduces adverse effects associated with propofol |
| Dose — 6–8 mg/kg IV without premedication |
| Dose — 2–4 mg/kg IV with premedication |
| Administer slow IV to effect over 60–120 seconds |
| Give half of calculated dose and await maximum effect, proceed further with incremental doses until desired effect if achieved |
| Alfaxalone |
| Always pre oxygenate |
| Consider premedication — 20–50% reduction in alfaxalone dose |
| Premedication reduces adverse effects associated with alfaxalone |
| Dose — 3 mg/kg IV without premedication |
| Dose — 2 mg/kg IV with premedication |
| Administer slow IV to effect over 60 seconds |
| Give one quarter of the calculated dose every 15 seconds |
Propofol and alfaxalone can cause excitatory signs during the induction process, such as myoclonus, paddling and nystagmus. Excitatory episodes can delay tracheal intubation. Premedication reduces the incidence of excitatory signs and all patients should receive adequate premedication. Occasionally, premedication does not prevent the occurrence of excitement during the anaesthesia induction period. Refractory excitement can be treated successfully with diazepam 0.2 mg/kg IV (Kastner, 2007). If a patient with BOAS is obese drug dosages should be calculated at the ideal weight, not the obese weight, to avoid over dosing.
Nursing support
Effective pre-oxygenation prior to anaesthesia will delay de-saturation in patients that are exposed to post-induction apnoea and at risk of delayed tracheal intubation because the functional residual capacity of the lungs will contain a higher percentage of oxygen. In healthy dogs, 3 minutes of pre-oxygenation via a facemask can delay de-saturation by 3 to 4 minutes (McNally et al, 2009).
If available, electrocardiography (ECG) should be connected prior to induction of anaesthesia. If not available, an alternative and effective method is to manually palpate a pulse or cardiac auscultation. While cardiac arrhythmias are rare, they can occur during induction of anaesthesia, especially during episodes of hypoxaemia. BOAS breeds can also have a high vagal tone and vagal stimulation associated with pharyngeal manipulation and ET tube intubation, can stimulate a vagal reflex (Wiederstein and Moens, 2008) and result in significant bradycardia and pos sible cardiac arrest. Atropine 0.02–0.04 mg/kg IV should be available for instances of vagal bradycardia.
Airway management
Tracheal intubation can prove difficult in all patients with varying severity of BOAS and tracheal intubation can be daunting. Often the mouth is too small small for the overly large tongue and the long soft palate prevents easy visualisation of the larynx. Everted saccules and laryngeal collapse can make ET tube placement difficult.
A number of things will help with ET intubation:
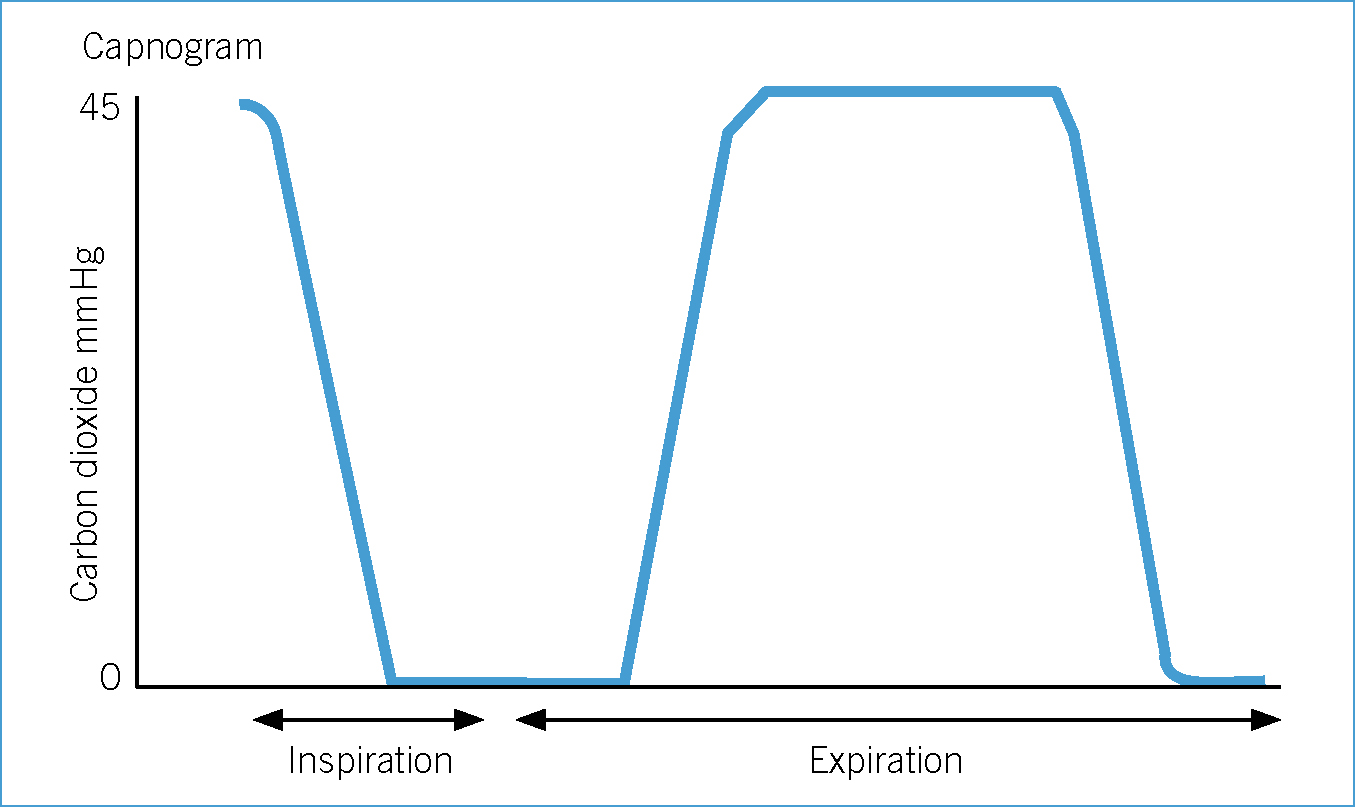
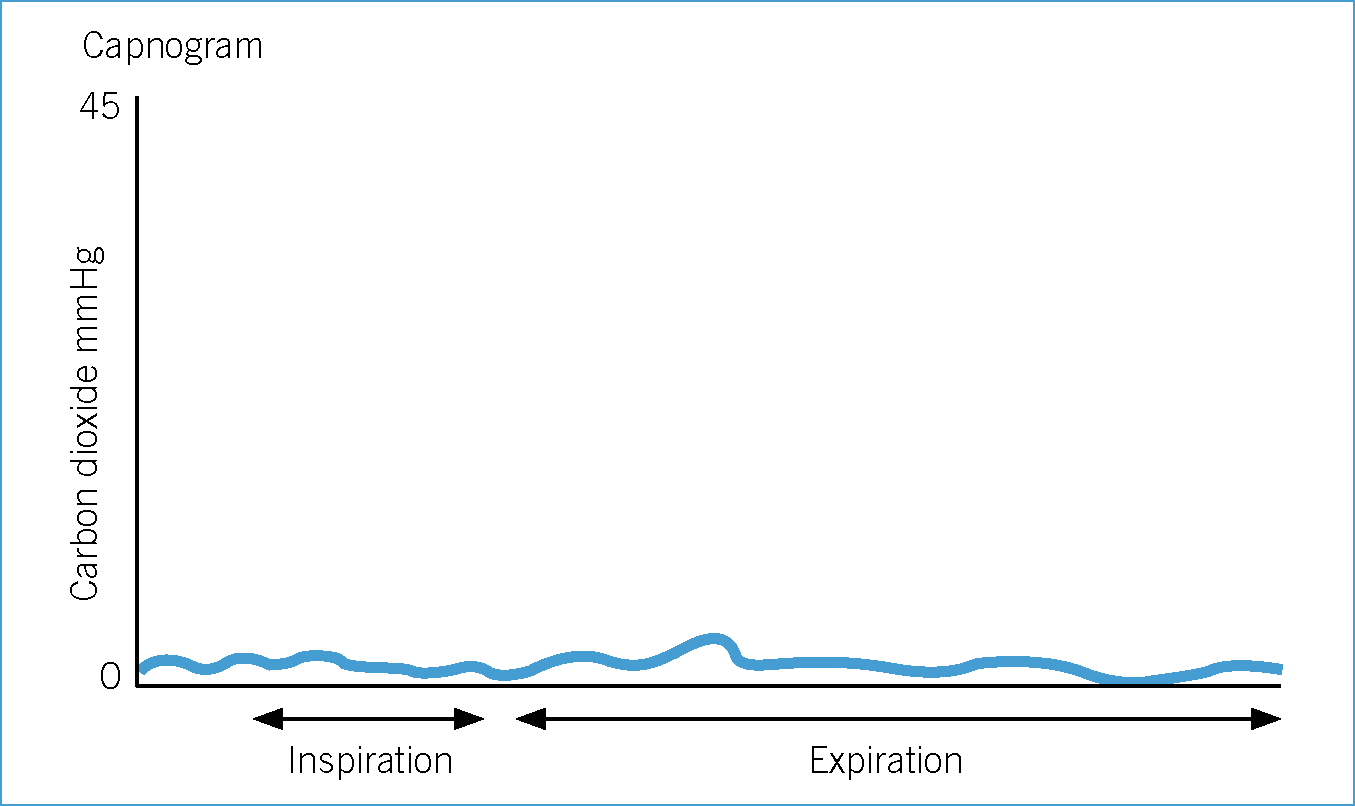
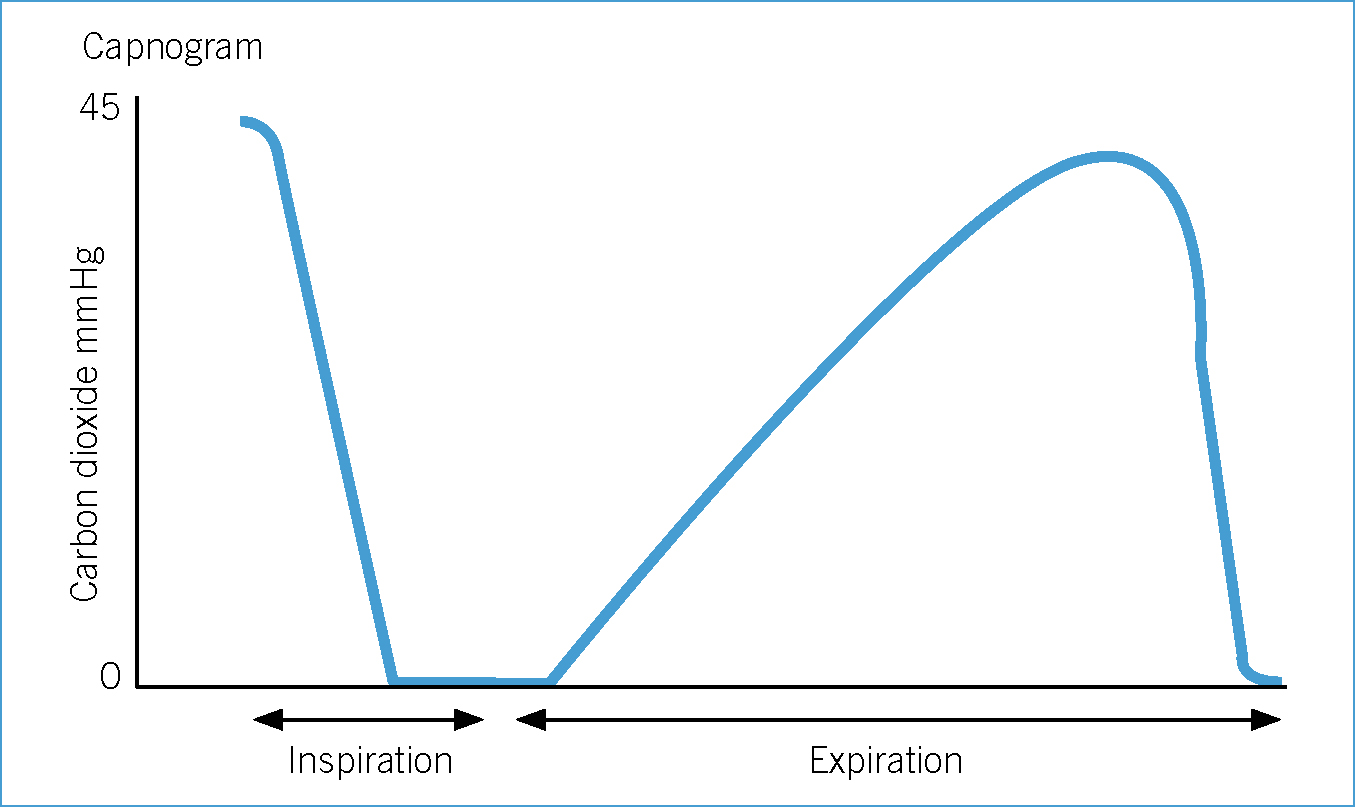

Hypoventilation and hypercapnia
Most anaesthetic agents can cause some degree of respiratory depression and most patients hypoventilate during anaesthesia. BOAS patients are particularly at risk of hypoventilation and hypercapnia during anaesthesia. The everted laryngeal saccules and laryngeal collapse prevent insertion of sizeable ET tubes and often tracheal intubation is performed using narrow ET tubes. Narrow ET tubes further decrease an already small trachea resulting in an increase in resistance to gas flow and exhalation.
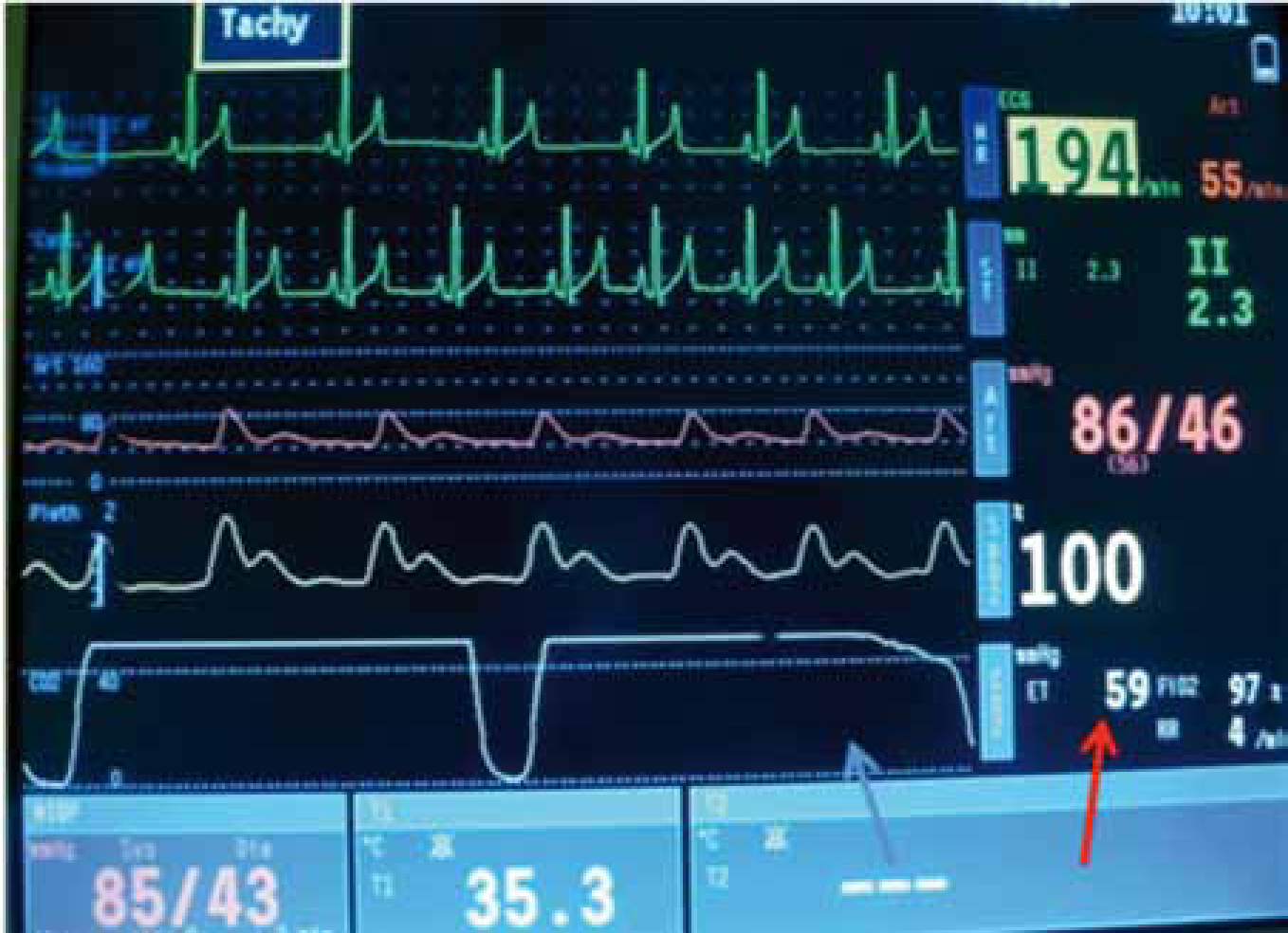
Normal end tidal carbon dioxide (ETCO2) is between 35–45 mmHg (Clark, 2009). Some veterinary surgeons would advocate initiation of assisted ventilation at levels above this range (Hammond, 2007). However, indications and the degree of hypercapnia at which to provide assisted ventilation remain controversial. In addition, some conscious BOAS patients already have an elevated PaCO2 compared with meso- or dolichocephalic dogs due to existing upper airway disease (Hoareau et al, 2012).
Mild hypercapnia is normally well tolerated in healthy patients (Hammond, 2007). However, in some patients even mild hypercapnia can cause adverse effects, for example acidaemia, hyperkalaemia, reduced myocardial contractility and ventricular arrhythmias. Further research is required in this area of clinical practice. Currently, the author of this article provides assisted ventilation when ETCO2 exceeds 50–55 mmHg. However, assisted ventilation should be provided earlier if adverse effects are noted.
Recovery and ET tube extubation
All anaesthetic monitoring should continue into the post-operative period — capnography, ECG, pulse palpation and pulse oximetry. The primary concern during recovery is airway obstruction once the ET tube has been removed and the most valuable piece of monitoring equipment available in clinical practice is the anaesthetist or nurse monitoring the patient. This person can visually observe respiratory pattern and assess for signs of dyspnoea, airway obstruction or cyanosis. At this point the patient is often moving and pulse oximetry can become unreliable.
ET tube extubation should be performed in a controlled environment. The patient should be recovered next to an anaesthetic machine and equipment for re-intubation and ventilation should be available; additional IV induction agent, ET tube, laryngoscope, oxygen and an anaesthetic breathing circuit should also be available. Smaller ET tubes may be necessary if laryngeal oedema has developed.
Prior to extubation the airway should be clear of fluid or debris that may have accumulated during the anaesthetic period. Suction or gauze swabs can be used to wipe away excess material. Patients at risk of regurgitation should be recovered in sternal recumbency with their head elevated to help avoid aspiration of regurgitant fluid.
Extubation should be postponed until the patient is swallowing and able to protect their own airway. Early extubation, while the patient is still sedated, will increase the risk for upper airway obstruction. Patients that are recovering slowly, and showing no signs of swallowing, should not be stimulated to provoke a swallowing reflex as partially anaesthetised patients can become re-sedated and unable to maintain airway patency (personal experience). Instead, the patient should be monitored closely and extubated once fully recovered from anaesthesia.
Supplemental oxygen and patient observation should continue until the patient is stable. Occasionally it may be necessary to pull the patient's tongue forward out of the mouth or open their mouth to clear the upper airway. The tongue should be continually assessed for signs of cyanosis and ideally the pulse oximeter should be used to assess saturation.
Regurgitation and gastro oesophageal reflux (GOR)
The BOAS patient is at risk of regurgitation and GOR due to increased abdominal effort or pressure associated with increased respiratory effort both during and post anaesthesia. Pre-emptive measures can be taken to reduce instances of GOR or regurgitation. The pre-operative administration of omeprazole, a proton pump inhibitor, at 1 mg/kg per os at least 4 hours prior to anaesthesia, has been shown to lower the incidence of gastric reflux in anaesthetised dogs (Panti et al, 2009). The incorporation of omeprazole into the anaesthetic plan may help to reduce instances of regurgitation during the anaesthetic period. However, oral administration of omeprazole may be difficult in a dyspnoeic patient; IV administration may be preferred. Omeprazole is unlicensed in dogs.
Other preventative methods can include
Other measures can be taken to reduce instances of regurgitation and GOR or prevent adverse sequelae, for example aspiration of gastric contents once regurgitation or GOR have occurred.
Once GOR or regurgitation has been noted, therapy should be started to reduce the potential clinical sequelae of oesophagitis and aspiration. The cuff on the ET tube should be checked to make sure no air leak is evident. The oral cavity should be examined, cleaned and any gastric fluid or material removed. Some practitioners also lavage and suction the oesophagus to remove gastric fluid (Bradbrook, 2011).
The use of gastroprotectants should be considered if regurgitation has occurred. Oral sucralfate and either an oral or IV histamine (H-2) receptor antagonist, such as ranitidine or cimetidine, are the most commonly employed medical treatment options. Cimetidine is the only licensed product. If there is concern the patient may have aspirated gastric contents, then appropriate antimicrobial therapy should be started.
Corneal ulcer formation
BOAS breeds are at high risk of ophthalmic problems due to breed conformation, prominent bulging eyes and decreased corneal sensitivity. During anaesthesia their eyes are often exposed to corneal drying and subsequent corneal ulceration. Ocular lubrication is recommended during the anaesthetic period and eye lubrication should be applied every 60-90 minutes during anaesthesia and 90-120 minutes post operatively.
Conclusion
BOAS patients can prove to be difficult during the anaesthetic period. Their breed conformation results in upper airway changes that can lead to upper airway obstruction and resultant cyanosis. Additional anaesthesia and nursing care is required in these patients.

