References
The regurgitating kitten
Abstract
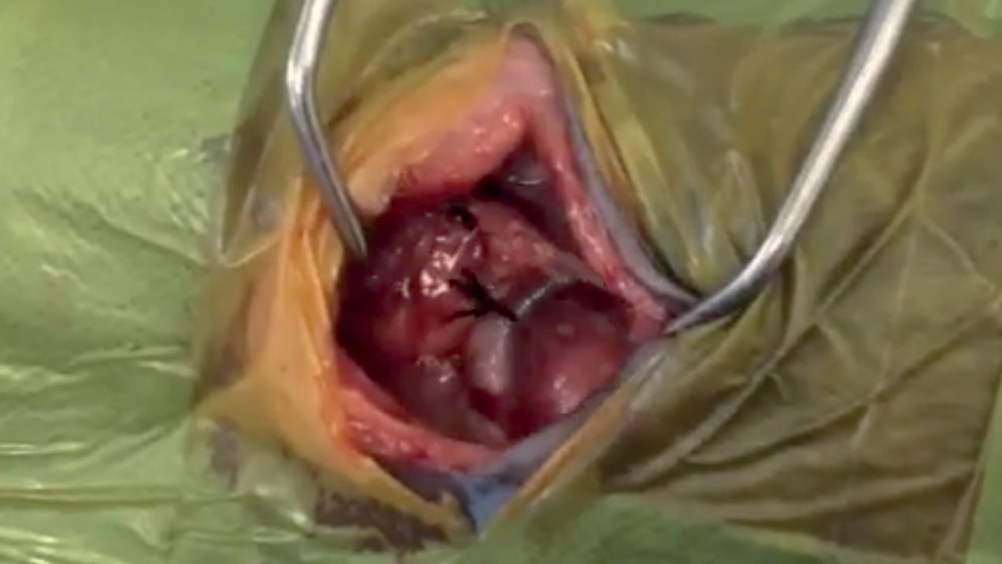
Vascular ring anomalies present in young patients and will inhibit growth and development. Therefore it is essential that these patients have the anomaly corrected as soon as clinically stable enough to undergo surgical correction. In particular the congenital malformation of the persistent right aortic arch (PRAA) is uncommon in practice to see, but does require good knowledge and understanding by the veterinary team to provide a successful outcome.
The patient presented to the practice with a 1 month history of regurgitation of food, and upper respiratory noise at the point of the cranial chest and at the thoracic inlet.
Differential diagnoses included:
The patient had been rescued from under a shed at approximately 4 weeks of age, and since then he had been hand reared. As growth occured, he developed regurgitation of his food, despite the owner feeding him from a height. He was vastly underweight for his age and was much smaller than his littermate. The referring veterinary surgeon (RVS) had radiographed the chest which revealed a megaoesophagus and that the aorta appeared to be on the right. On chest auscultation there was upper respiratory noise present at the thoracic inlet. The owner reported no coughing and the patient had been eating and drinking well. All other vital signs had no abnormalities. An intravenous (IV) 22 g catheter was placed in the right cephalic vein.
Register now to continue reading
Thank you for visiting The Veterinary Nurse and reading some of our peer-reviewed content for veterinary professionals. To continue reading this article, please register today.

