Toxoplasma gondii is a single-celled coccidian parasite. Coccidia in general have very complicated life cycles, with most of them being host-specific and transmitted via the faeco-oral route. Toxoplasma gondii is one of the most important parasites of animals, with felids as the definitive hosts and warm-blooded animals as intermediate hosts (Figure 1). It has three infectious stages: tachyzoites (in groups), bradyzoites (in tissue cysts) and sporozoites (in oocysts). It can also be transmitted through the consumption of infected meat and vegetables, as well as vertically (Frenkel et al, 1970; Dubey, 2010).
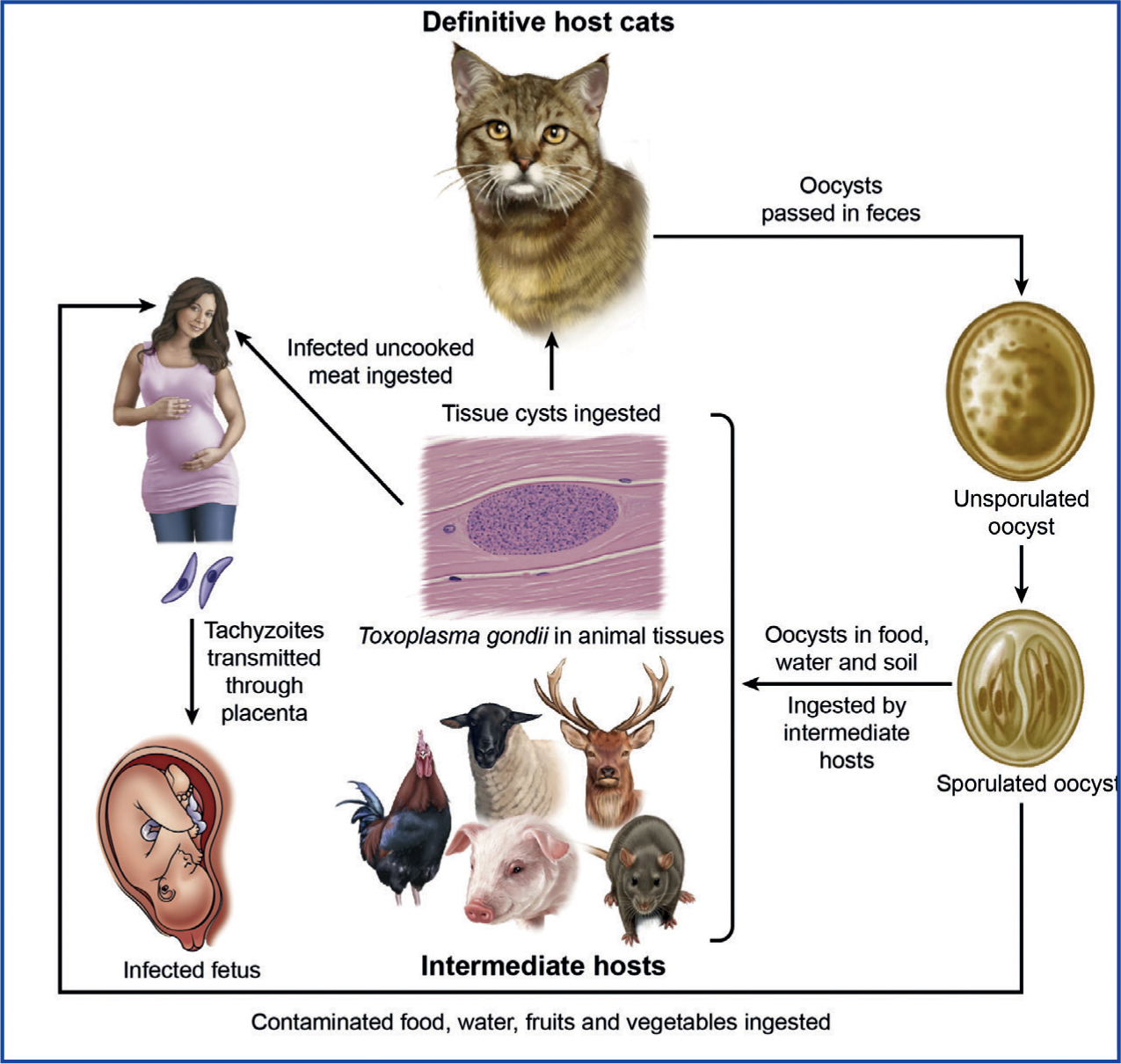
Toxoplasma is an obligate intracellular parasite, infecting most nucleated cells of warm-blooded animals. This parasite (toxon=arc, plasma=form) derives its name from the crescent shape of the tachyzoite stage (Figure 2a), (the approximate size of an erythrocyte, 2 x 6 μm), and gundi from Ctenodactylus gundi, the North African rodent from which this parasite was first isolated in 1908 by Nicolle and Manceaux (Dubey, 2008).
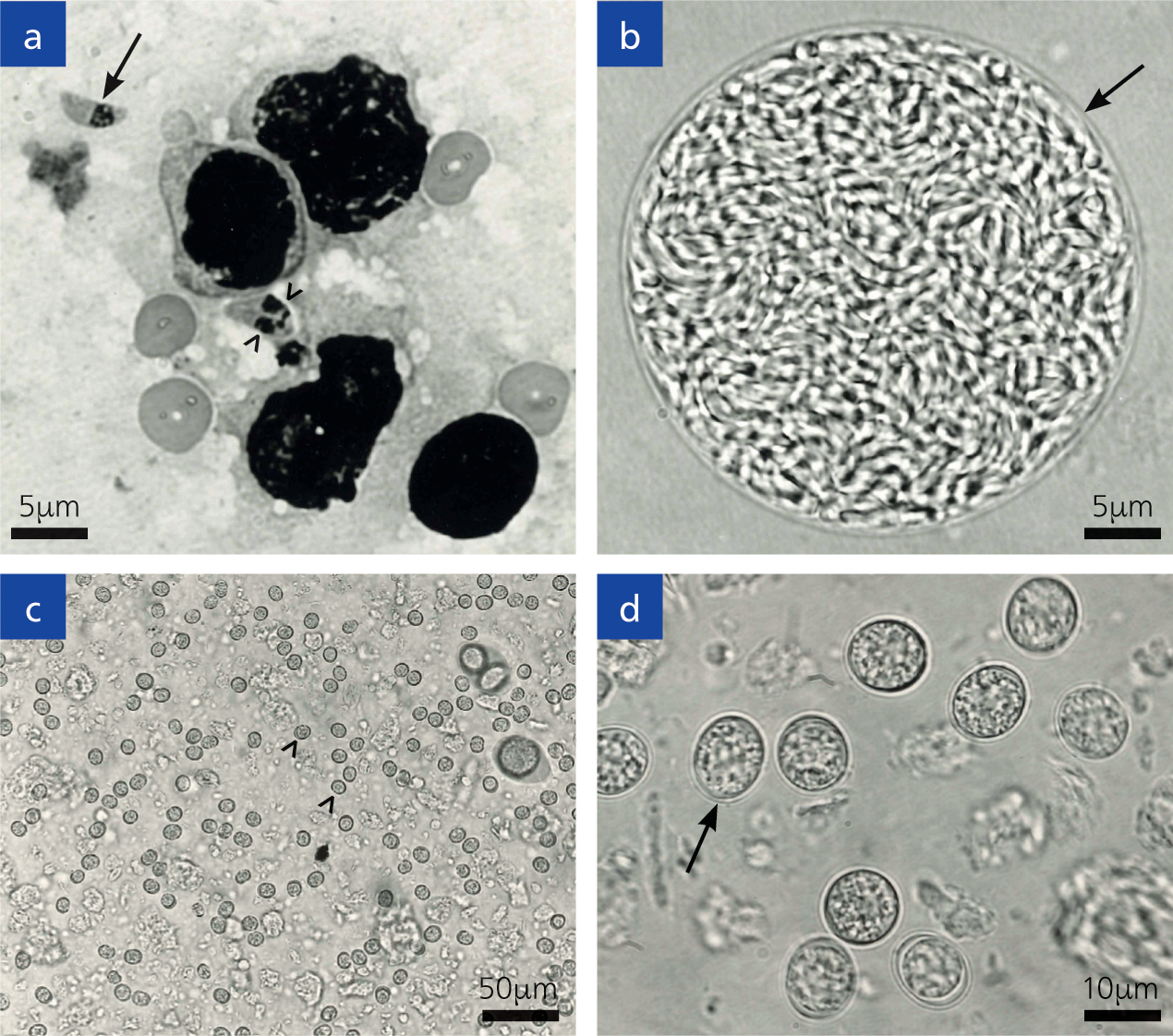
Tachyzoites can multiply in any cell of the body; soon after its invasion it reproduces asexually inside the host cell. Replication continues until the cytoplasm of the cell bursts. After a few replicative cycles, tachyzoites convert to bradyzoites that are encysted in many tissues. Tissue cysts remain microscopic (<100 μm). The tissue cyst has an elastic, thin wall and can encompass up to hundreds of the crescent moon-shaped stage known as bradyzoites (Figure 2b). Bradyzoites have an approximate size of 7 x 1.5 μm. Tissue cysts can develop in visceral organs, however they are more commonly found in the brain and eye, in addition to skeletal and cardiac muscle. Tissue cysts are the terminal stage of the life cycle in the intermediate host and are immediately infectious. On ingestion of tissue cysts, the cyst wall is dissolved immediately by juices in the gut, but bradyzoites can survive long enough to infect host cells.
Felids can acquire the infection by ingesting either the sporulated oocysts from the environment or infected tissues. However, epidemiological data indicate that cats become infected mostly postnatally when they begin to prey, soon after weaning. The bradyzoites are released from the tissue's cysts in the gut, penetrate the epithelial cells of the small intestine and begin the development of numerous generations of asexual and sexual cycles (Dubey and Frenkel, 1972). The stage in the epithelial cell is called a schizont, and the organisms released from them are called merozoites and these form male and female gametes. Following fertilization, the female gamete starts forming the oocyst wall, and ensuing maturation, they are discharged into the intestinal lumen after the rupture of the intestinal epithelial cells and are then excreted into the environment as unsporulated oocysts (Figure 2c, d).
The prepatent period (days before oocysts are excreted in faeces) in felids is 3–10 days after ingesting tissue cysts and 19 days or more after ingesting tachyzoites or sporulated oocysts (Dubey, 2006). Millions of oocysts can be excreted in a few days. Cats usually remain asymptomatic during the oocyst excretion phase. Formation of oocysts is only achieved in felids (domestic or wild). Oocysts are very resistant to harsh environmental conditions and sporulate after 1–5 days depending on aeration and temperature, becoming infective to humans and other mammals as well as avians. This oocyst-excretion phase is followed by development of immunity in cats. The relatively short oocystexcretion phase and the length of immunity explain why oocysts have been detected in faeces of 1% of cats at any time surveyed. Seroprevalence in felids can vary with age and lifestyle, with higher values found in strays compared with house cats (Dubey and Jones, 2008). In felids, the infective stage can determine how effective the parasite can be at reproducing, as less than 50% of felids excreted oocysts when they ingested tachyzoites or oocysts. In comparison, nearly all cats (81%) that ingested tissue cysts excreted oocysts (Dubey and Frenkel, 1972; Dubey, 2006). This parasite can dwell in extra-intestinal tissues of felids for a long time, and even for the rest of its life.
Clinical importance in animals
Although T. gondii can infect virtually all warm-blooded hosts, clinical outcome differs. For example, cattle and horses are more resistant than sheep and goats. A few examples are cited here in different groups of hosts.
Companion animals
Among domestic animals, toxoplasmosis was first reported in dogs in 1910 (Dubey, 2008). A century later, toxoplasmosis is considered rare in dogs and occurs mainly in dogs not vaccinated against distemper virus or those treated with immunosuppressive drugs. When it does occur, pneumonia and hepatitis are the most common presentations. Many of the clinical signs previously attributed to toxoplasmosis in dogs in the past are now attributed to neosporosis, and are caused by the related parasite, Neospora caninum (Dubey et al, 2017)
Cats
Although any organ can be affected, with associated clinical signs, pneumonia is the most serious and is usually fatal if not treated. Toxoplasmosis is most serious in congenitally affected kittens but it can be fatal in cats of any age (Dubey 2010). Cats are the most important hosts in the epidemiology of toxoplasmosis and their zoonotic importance is discussed later (Figure 3).

Livestock
As stated earlier, there are no confirmed cases of clinical toxoplasmosis in horses and cattle. Abortion is the main clinical sign in sheep and goats worldwide, and millions of lambs are still lost yearly to toxoplasmosis.
In pigs, clinical signs are rare but it can cause premature births, neonatal death and pneumonia, with rare reports of myocarditis and encephalitis in most parts of the world (Dubey and Jones, 2008). However, epizootics of toxoplasmosis have been reported from Japan and China (Dubey, 2010; Wang et al, 2017). Clinical toxoplasmosis in domestic poultry is rare, but has been reported with concurrent Marek's disease.
Wild and zoo animals
New World primates, Australasian marsupials (kangaroos), and passeriformes (canary and finches) in captivity are highly susceptible to toxoplasmosis. Squirrel monkeys (Saimiri spp.) can die acutely with no prior signs. Severe ocular toxoplasmosis has been reported in finches, even affecting the globe. Pallas cats (Otocolobus manul; natural habitat is Himalayas) are difficult to maintain in zoos because of perpetual toxoplasmosis. Pallas cats also can excrete T. gondii oocysts that can contaminate the environment in zoos.
Zoonotic potential
Approximately, one-third of humanity is infected with T. gondii worldwide (Robert-Gangneux and Dardé, 2012). In most infected humans, toxoplasmosis is asymptomatic. However, it can be serious in those infected in utero and in those that are immunosuppressed. Even immunocompetent adults can die of toxoplasmosis. If a woman not previously exposed to T. gondii acquires infection during pregnancy, there is a 50% chance of transplacental infection. Time of infection during the pregnancy period can determine the outcome. If the infection is during the first trimester of pregnancy, this can lead to abortion, death or serious impairment of the fetus, such as retinochoroiditis, endocranial calcification, hydrocephaly, and microcephaly (Jones et al, 2010). In contrast, if the infection is during the second or third trimester, the signs are subclinical, although retinochoroiditis and neurological disorders are sometimes present. The disease can recrudesce due to persisting tissue cysts. Ocular disease is the most common sequelae of congenital toxoplasmosis. Although asymptomatic at birth, infections can flare up as many as 20 years later (Dubey, 1976).
The socioeconomic impact of this disease in people suffering from it, and the cost of care for sick children, especially those with mental retardation and blindness, are enormous (Stillwaggon et al, 2011). Therefore, in some European countries such as France and Austria, the testing of all pregnant women for toxoplasmosis is routine (SYROCOT, 2007).
Facts about toxoplasmosis transmission and cats
- Cats of any age can excrete T. gondii oocysts.
- Cats are infected in nature by preying on infected animals or eating infected meat.
- Millions of oocysts can be excreted within a few days.
- Oocysts are environmentally resistant and are not killed by disinfectants that will not harm humans.
- Cats are usually asymptomatic when excreting oocysts.
- There are no drugs that can eliminate infection in cats.
- There are no tests to diagnose if a cat is safe with respect to transmission. Cats that have excreted oocysts once can do so again (Dubey, 1976; 1995; Zulpo et al, 2018)
- Oocysts are not present on fur of cats. Thus, T. gondii infection is not contracted by petting a cat.
- Ownership of cat is not associated with infection in humans. The culprit is infected faeces and not the cat.
- To prevent infection, cats should never be fed uncooked meat.
- There is no safe disposal of infected faeces or cat litter. Flushing down the toilet is not recommended. Currently, sealing in a plastic bag and disposing of it in garbage is one way to dispose of faeces with a hope that heat generated in the disposal process kills oocysts.
- Cat litter trays should be disinfected daily with boiling water.
- Mask and gloves should be worn while handling sick pets.
Diagnosis
Diagnosis can be carried out using biologic, serologic or histological methods. Fluids from the thoracic cavity of aborted fetuses, milk, and extracts of infected tissue or liquid drained from muscles that have been frozen and thawed are all acceptable samples (Fricker-Hidalgo et al, 2009). Detection of humoral antibodies can be achieved by several procedures, such as the Sabin-Feldman dye test (DT), modified agglutination test (MAT), indirect fluorescent antibody assay (IFA), direct agglutination test, latex agglutination test (LAT), enzyme-linked immunosorbent assay (ELISA) and the immunosorbent agglutination assay test (IAAT). The IFA, IAAT and ELISA have been modified to detect IgM antibodies, as these appear much faster after infection, compared with the IgG antibodies following recovery (Remington et al, 2011).
Treatment
Sulphadiazine and pyrimethamine are two drugs widely used (Remington et al, 2011). These drugs are beneficial to halt the active multiplication of the parasite. However, they will not eradicate the infection. Other drugs such as atovaquone, spiramycin, and clindamycin have also been used to treat difficult cases.
Prevention
- People handling meat should thoroughly wash their hands with soap and water before they perform their tasks, as well as washing all their utensils.
- Good hygiene is a very effective measure as the stages of this parasite present in meat are killed by the contact with soapy water (Dubey and Jones, 2008).
- Toxoplasma stages in meat are killed with temperatures above 67°C, and by freezing to -13°C (Dubey, 2010). Overnight freezing in a household freezer is effective in killing Toxoplasma tissue cysts.
- Pet cats should be fed only dry, canned or cooked food and the litter box emptied daily, but not by a pregnant woman.
- At present, there is no vaccine available for prevention of toxoplasmosis in humans.
- Cats in zoos should be fed only meat that has been frozen overnight or cooked.
Conclusion
To prevent human infection, all meat should be cooked well before consumption and gloves should be worn while gardening to prevent exposure to soil contaminated with T. gondii oocysts excreted in cat faeces. Pregnant women should avoid contact with cat faeces, soil and raw meat. This does not mean banning pet cats from their homes.
KEY POINTS
- Approximately, one-third of humanity is infected with Toxoplasma gondii worldwide.
- Cats of any age can excrete T. gondii oocysts.
- Diagnosis can be carried out by biological, serological or histological methods.
- Sulphadiazine and pyrimethamine are two drugs widely used to inhibit the active multiplication of the parasite.
- To prevent human infection, all meat should be cooked well before consumption and gloves should be worn while gardening to prevent exposure to soil contaminated with T. gondii oocysts excreted in cat faeces
- Pregnant women should avoid contact with cat faeces, soil and raw meat.


