References
Arterial lines: why, when, how?
Abstract
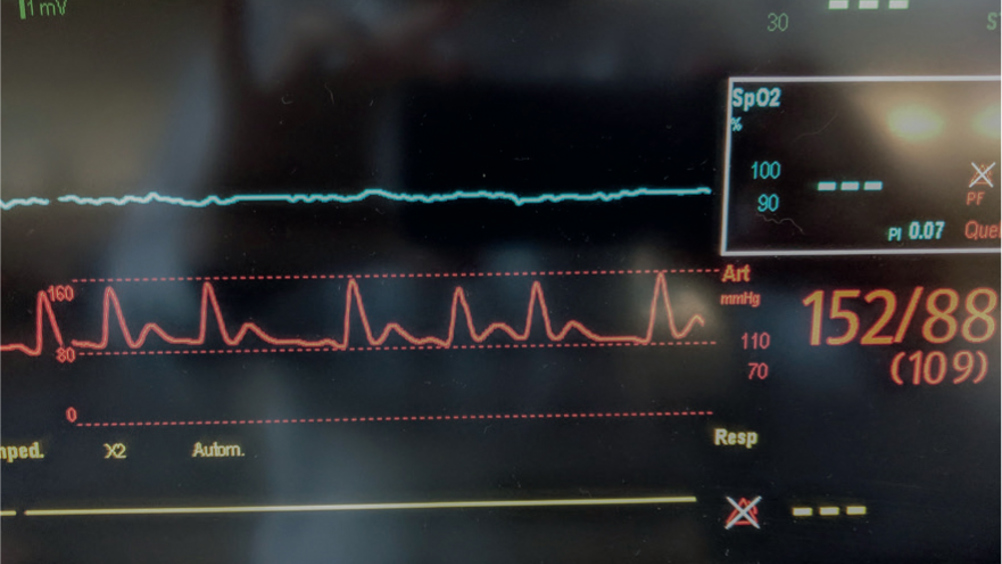
Arterial lines are a useful tool in the emergency and intensive care patient. Invasive blood pressure measurement is considered the gold standard in patient care and has become commonplace in veterinary practice, especially in veterinary anaesthesia. Arterial catheters are catheters placed within the arterial lumen by a skilled veterinary surgeon or veterinary nurse. This catheter is attached to a monitor which provides information such as systolic, mean and diastolic arterial pressure, heart rate and an arterial waveform. Having an invasive arterial catheter gives the advantage of a constant measurement of arterial blood pressure, meaning the effects on patients of drugs, fluids and environment can be seen. Arterial lines are useful in patients where rapid changes in blood pressure are anticipated, and abnormal measurements can be detected quickly. This article discusses the different aspects of arterial lines from what they are, to waveform analysis and complications.
Placing an arterial line is an invasive procedure, it can be painful and uncomfortable for the patient and presents other risks, as discussed in this article. It is, however, a valuable tool in intensive medicine and provides benefits such as continuous blood pressure (BP) measurement and arterial blood gas sampling. Benefits and risks of using non-invasive blood pressure (NIBP) versus invasive blood pressure (IBP) monitoring techniques need to be considered with each patient. This article reviews IBP monitoring, including: how to place an arterial line; waveform analysis; complications; troubleshooting and comparison to NIBP (Figure 1).
IBP is an invasive and direct measurement technique. An arterial line is a catheter that is placed into an artery. This is attached to compatible tubing which is connected to a monitor via a transducer. This allows the measurement of IBP, observation of the arterial waveform and sampling of arterial blood gases.
BP measurement, whether direct or indirect (via oscillometric, high definition oscillometric (HDO) or ultrasonic Doppler flow), is defined by the measurement of pressure of blood flow on the vessel wall. The unit of measurement is millimetres of mercury (mmHg). Systolic arterial pressure (SAP) is the measurement of pressure during cardiac systole (contraction). Diastolic arterial pressure (DAP) is the measurement of pressure during cardiac diastole (relaxation). The mean arterial pressure (MAP) is calculated from the SAP and DAP.
Register now to continue reading
Thank you for visiting The Veterinary Nurse and reading some of our peer-reviewed content for veterinary professionals. To continue reading this article, please register today.

