Routine flea control should be included as part of any general health protocol for dogs and is an essential part of the ongoing management of dogs with pruritic skin disease. In order to be able to advise on suitable ectoparasiticides, it is important that the veterinary nurse has an appreciation of the life cycle of the cat flea (the principal flea to affect the dog) and the speed of action and persistence of the most common of the licensed veterinary products that can be used as part of an integrated control programme.
Flea biology
The cat flea, Ctenocephalides felis, belongs to the Order Siphonaptera and the Family Pulicidae. Despite its name the cat flea is recognised as the most common and important ectoparasite of domesticated dogs worldwide (Dobler and Pfeffer, 2011; Rust, 2017). A study in the UK recruited 662 dogs from 326 practices and found that 14.4% of dogs were infested and of those fleas 90% were cat fleas (Abdullah, 2019). Fleas are ubiquitous and exposure if difficult to avoid (Figure 1). Numerous reviews on the biology of C. felis have been published over the last 20 years (Dryden and Rust, 1994; Rust and Dryden, 1997; Blagburn and Dryden, 2009; Dryden et al, 2011; Iannino et al, 2017) and the reader is referred to these for a more in-depth description of the flea life cycle.

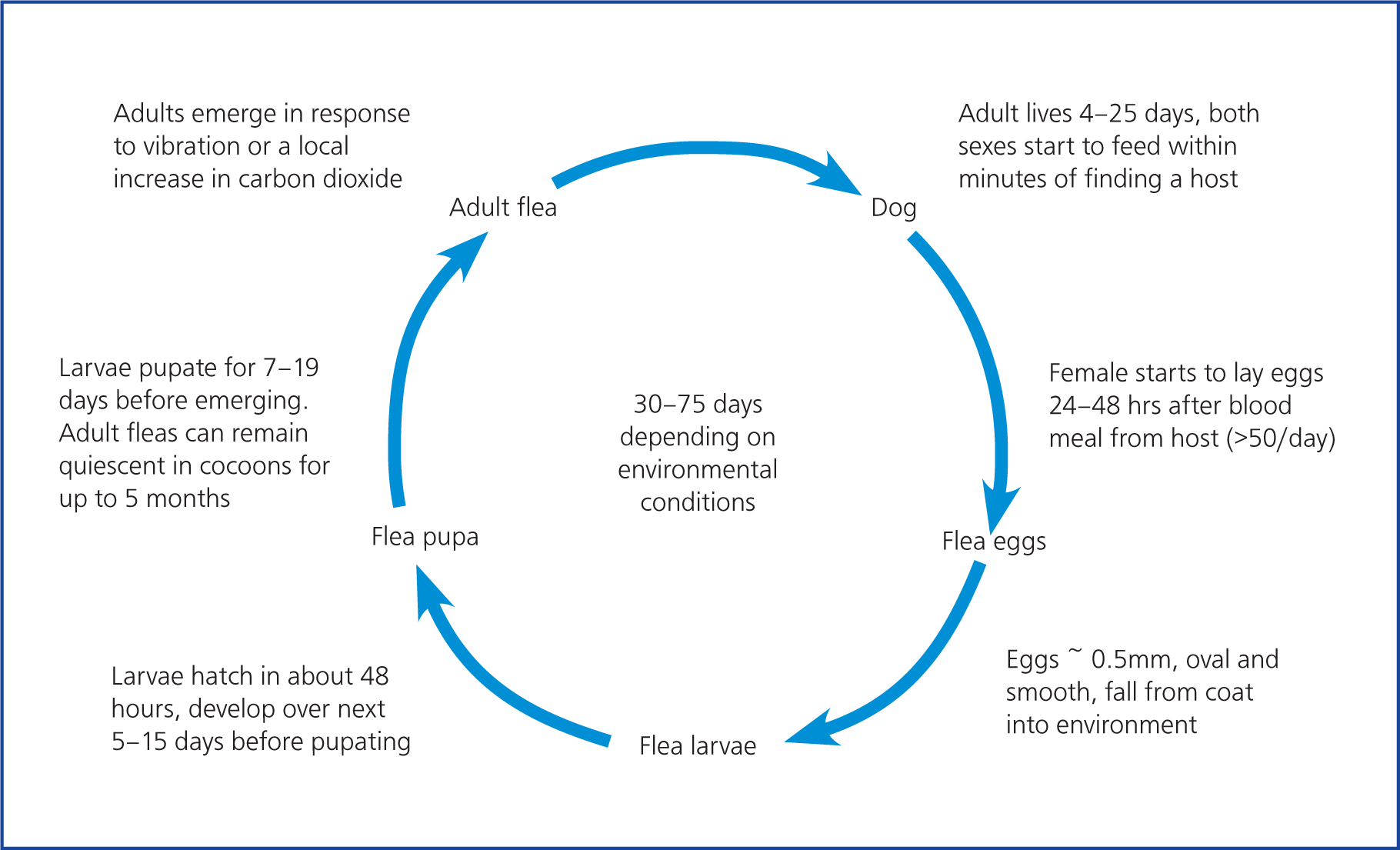
The life cycle of the flea (Figure 2) starts as the adults emerge from their cocoons in response to vibration or a local increase in carbon dioxide (Dryden and Rust, 1994; Rust and Dryden, 1997). As flea eggs, larvae and pupae are not found on the animal but accumulate around the animal's feeding and resting places (Beck et al, 2006), the newly hatched adults do not have very long to wait for a host to come along. The adult cat flea is 1–3 mm in size, reddish brown to black in colour and wingless and usually lives 4–25 days. It relies on its powerful hind legs to jump onto their hosts and for movement through the fur (Dryden and Rust, 1994; Rust and Dryden, 1997). C. felis remains on the host and does not, contrary to popular opinion, jump from animal to animal (Rust, 2017). Adult fleas require a fresh blood meal to mate and start egg laying. Fleas start to feed within minutes of finding their host and egg laying starts 24–48 hours later (Dryden et al, 2011). The female flea can lay 50 eggs a day (Rust, 2017). Eggs are oval smooth and about 0.5 mm in size and readily fall out of the dog's coat into the immediate environment. Larvae hatch within about 48 hours and develop, depending on the temperature and humidity, over the next 5–15 days. They are photophobic and geotropic, so will tend to burrow deep into carpets and bedding where the relative humidity and temperature is higher (Dryden and Rust, 1994; Rust and Dryden, 1997). They feed on organic material in their vicinity, especially dried adult flea faeces, which are rich in undigested blood from the host animal. The larvae spin a silk cocoon to undergo final metamorphosis to emerge as adults (Dryden and Smith, 1994). The life cycle usually lasts 30–75 days depending on ambient temperature and humidity (Dryden and Rust, 1994; Rust and Dryden, 1997; Rust, 2017).
Fleas and canine skin disease
All dogs will react to flea bites but where individuals are not sensitised to flea bites the irritation is generally transient and does not lead to clinical signs. Where dogs are sensitive to components of the flea's saliva injected intradermally during flea feeding, they will develop much more severe and widespread clinical signs. Canine flea allergic dermatitis (FAD) principally affects the caudo-dorsal lumbosacral area often with extension of signs onto the dorsal tail head, caudomedial thighs, abdomen and flanks. Despite this typical presentation, flea allergy can occur concurrently with other allergies which blurs the clinical signs. There seems to be a close association between dogs with FAD and atopic dermatitis (AD). It has been shown that dogs in flea endemic areas are four times more likely to suffer from FAD and AD rather than FAD alone (Sousa and Halliwell, 2001). Such evidence would suggest that AD predisposes to the development of FAD, reinforcing the need for an integrated approach to control of the atopic dog, which should include good flea control rather than merely using symptomatic anti-pruritic therapy.
Identifying the presence of fleas
In all dermatology cases taking a thorough history can help raise the suspicion of a flea component to a skin problem. Such facts as a history of flea exposure (there may be stray cats in the garden or the dog has rolled on a dead hedgehog), can provide an initial clue. The lack of appropriate flea control, such as the use of a product without any proven activity against fleas (garlic and lemon are commonly but mistakenly, cited as good flea controls); the use of a product on an infrequent base; the application of the wrong size of product relative to the dog's weight or the use of a flea product in combination with shampoo therapy, which may render some topical products less effective, may all suggest likely presence of fleas. Where such historical evidence raises the possibility of a flea problem and clinical signs are consistent with fleas, then specific diagnostic tests can be undertaken to identify the presence of fleas and/or flea faeces.
Finding fleas on a dog can be challenging. They are dark brown to black, wingless insects with a laterally compressed body, that range in size from 1.5–3.5 mm, which is about the size of the end of a ballpoint pen tip. Their body outline is sometimes described as being ‘avocado’ shaped. Running a fine toothed flea comb through the coat may trap fleas and flea faeces (Figure 3) however the flea's powerful legs and streamlined bodies allows them to move rapidly through the pelage to avoid detection. Often turning the dog over onto its back (with care) will surprise fleas on the ventrum, which can be trapped by catching them on a piece of acetate tape. If you can catch a flea the author would always recommend showing it to the client. It is a powerful incentive to any client to invest in rigorous flea control. Microscopic examination of the flea can be useful to identify the species and hence the source of infestation. There are excellent resources that outline the key identification factors for each flea (Bitam et al, 2010; Dobler and Pfeffer, 2011). The most useful pointers are the presence, size and arrangement of the genal and pronotal combs (or spines). The genal combs are a row below the head, the pronotal combs a row behind the head. C. felis has both sets of combs present and the first two spines on the genal combs are the same length, unlike the dog flea C. canis where the first spine on the genal comb is half the length of the second. Most of the fleas found on dogs are C. felis, but occasionally C. canis can be found. As fleas are not host specific wildlife fleas such as Spilopsyllus cuniculi (rabbit flea) and Archaeopsyllus erinacei (hedgehog flea) can also be identified especially on outdoor dogs. Flea faeces can be seen with the naked eye especially if the infestation is severe. They appear as fine, black, crumbly grains within the coat, particularly on the dorsum. In milder cases or where flea numbers are small and clinical signs more marked due to an allergic component to the disease, a wet paper test or acetate tape impression smear are useful to assess for flea faeces. A wet paper test is performed by combing the dog's coat onto a piece of good quality, wetted white paper (Figure 3). Blood containing flea faeces produce dark red/brown streak on the paper as the blood dissolves out. Alternatively a piece of clear acetate tape can be pressed repeatedly onto the coat to pick up flea faeces and then mounted sticky side down on a microscope slide for examination (Figure 4). The faeces appear as comma shaped structures under magnification.


Finding fleas and/or flea faeces is enough evidence to recommend the institution of flea control, however even if investigative tests fail to reveal direct evidence of fleas, if the clinical signs and history are suggestive of fleas the author would recommend flea treatment. Where owners bathe a dog before presentation to the veterinary surgery or where a dog swims or grooms excessively the tell-tale signs of fleas can be lost.
Flea control
In the last 20 years a wide range of insecticides have been registered as flea products either as mono or combined therapies. Most of the actives such a fipronil, imidacloprid, lufenuron, methoprene, permethrin and pyriproxyfen registered prior to 1997 are still available and, despite anecdotal reports suggesting otherwise, resistance to these products is uncommon. According to the Arthropod Pesticide Resistance database there have to date only been reports of C. felis resistance to organochlorines, carbamates, organophosphates, pyrethroids and pyrethrin. Drugs such as fipronil and imidacloprid have been rejuvenated by being released as generics; others have benefited from changes in formulation, application technology and by being combined with other actives (Rust, 2017). Most insecticides licensed for veterinary use are available as POM-V products. Drugs such as mono formulations of imidacloprid and fipronil are available without veterinary prescription as NFA-VPS. Despite the availability and undoubted efficacy of these older products, new active ingredients continue to be developed driven by such factors as owner convenience, safety and cost.
Which flea product to choose?
Current standards set by the European Medicine Agency suggest that an adulticide must kill 95% of fleas within a designated time period to be licensed. Therefore as all licensed flea products have by definition a high degree of efficacy, recent studies on flea products have focused on the persistence of a product's adulticide activity between applications and the speed with which a product kills the fleas. While speed of kill and persistence are important, other factors such as owner compliance (tablet versus spot-on or collar), length of action (28 versus 84 days) and spectrum of ectoparasiticide activity should all be considered when prescribing a flea product. In an ideal situation a product that is administered or applied monthly should have the same 95% kill rate at 4 weeks that it has at 8 hours, so that an animal has identical levels of protection throughout the whole treatment period. The rationale behind speed of kill (SOK) is that the more quickly a flea is killed the more effectively the signs of a FAD should be controlled. Ideally a flea should be killed as soon as it alights on an animal so that the flea does not bite. However as feeding starts almost immediately after the host has been found, this is in practice very difficult to achieve. Current data would suggest that the primary role of any insecticide in managing FAD is to rapidly reduce flea numbers and reduce flea feeding rather than preventing bites (Dryden, 2009).
Adulticides
An extensive discussion of the mode of action, persistence and speed of kill of all the current licensed flea adulticides (Table 1) is beyond the scope of this paper, the reader is referred to other texts for a more detailed review than the one that follows (Rust, 2017; Dryden et al, 2011).
Table 1. Most important groups of flea adulticides and insect growth regulators (IGRs)
| Mode of action | Examples | Canine formulation |
|---|---|---|
| Chloride channel activators | ||
| Avermectin are agonists to glutamate-gated chloride channels in flea causing blockage of neurotransmission and paralysis | Selamectin | Spot-on treatment |
| Chitin biosynthesis inhibitors (juvenoid/IGR) | ||
| Benzoylphenylurea derivative that inhibits flea development through blocking chitin production and egg hatch | Lufenuron | Tablet with and without milbemycin oxime |
| GABA-gated chloride channel antagonist | ||
| Phenylpyrazole that blocks invertebrate GABA and glutamate gated chloride channels needed for inhibition of nerve impulses leading to hyperexcitability and neurotoxicity | Fipronil | Spot-on or sprayavailable as numerous different products |
| Isoxazoline potent antagonist of invertebrate GABA and glutamate-gated chloride channels (receptor binding more potent than fipronil) | Afoxolaner | Tablet |
| Fluralaner | Tablet and spot-on | |
| Lotilaner | Tablet | |
| Sarolaner | Tablet | |
| Juvenile hormone mimetic (juvenoid/IGR) | ||
| Mimics of juvenile hormones to prevent larvae from completing metamorphosis | Pyriproxyfen | Spot-on, shampoo, topical spray, collars and environmental spray |
| Methoprene | Spot-on, shampoo, topical spray and environmental spray | |
| Nicotinic acetylcholine receptors agonist | ||
| Furanicotinyl and chloronicotinyl (neonicotinoids) insecticides binds to post synaptic nicotinic acetyl choline receptors resulting in inhibition of cholinergic transmission and paralysis of parasites | Dinotefuran | Spot-on |
| Imidocloprid | Spot-on or a combination therapy with other actives or with flumethrin in a collar | |
| Nitenpyam | Tablet | |
| Spinosyn derivatives activate nicotinic acetyl choline receptors. Causes hyperactivity due to disrupting binding of acetyl choline in nicotinic acetyl choline receptors at post synaptic cell that prevents repolarisation of the cell | Spinetoram | Spot-on (cat only) |
| Spinosad | Tablet | |
| Sodium channel modulator | ||
| Pyrethroid group of drugs that work by prolongation of opening of voltage-gated sodium channels in invertebrates, resulting in increased action potentials and neurotoxicity | Cyphenothrin | Spot-on also in combination with other products |
| Deltamethrin | Collar | |
| Etofenprox | Shampoo (licensed for ticks), spray and environmental spray and fogger | |
| Flumethrin | Collar combined with imidacloprid | |
| Permethrin | Spot-on also in combination with other products, topical and environmental spray | |
| Phenothrin | Spot-on also in combination with other products | |
| Voltage-dependent sodium channel blockers | ||
| Inhibits sodium ion entry into nerve cells which results in impaired nerve function, cessation of parasite feeding, paralysis and death | Indoxacarb | Spot-on also available combined with permethrin |
Chloride channel activators
The principal flea adulticide within this group is selamectin. Selamectin has a broad spectrum of activity against both endo and ectoparasites and has been demonstrated to be safe and highly effective in the control of naturally acquired flea infestations on dogs and cats presented as veterinary patients in Europe (Benchaoui et al, 2000). It has also been shown to be highly effective in the treatment and prevention of flea infestations, without the need for supplementary environmental control measures (Shanks et al, 2000). However its SOK is inferior to some licensed adulticides (Schenker et al, 2003).
Nicotinic acetylcholine receptor agonists (NARAs)
This group of drugs includes the neonicotinoids (dinotefuran, imidocloprid and nitenpyam) as well as the spinosyn derivatives (spinosad, spinetoram). All of the products in this group have a rapid SOK. Both nitenpyam and imidocloprid have a superior SOK to both fipronil and selamectin (Schenker et al, 2003). A study by Blagburn (2010) assessed the SOK of spinosad and also its ability to reduce eggs counts. Reductions in mean flea counts were 85.5% at 2 hours and 100% at 4 hours demonstrating a rapid SOK which in turn led to a >99% reduction in egg production (Blagburn et al, 2010).
Voltage dependent sodium channel blockers
Indoxacarb is the flea adulticides within this class of drugs. Indoxacarb has excellent adulticide activity against C. felis to control flea infestation and clinical signs in a high proportion of dogs with FAD without the need for other drugs (Fisara et al, 2014).
Sodium channel modulators
This group of drugs contain actives such as permethrin, deltamethrin and flumethrin. The latter two drugs are usually used as a component of ectoparasiticide collars and both actives have good activity against fleas (Horak et al, 2012). However their onset of action is slow (Horak et al, 2012). Permethrin combined with either fipronil or with dinotefuran and pyriproxyfen as a spot on have been shown to be effective as a flea control products and possess a rapid SOK (Beugnet et al, 2015).
GABA gated chloride channel antagonists
This group of drugs contains the older phenylpyrazoles which includes fipronil and the newer class of isoxazolines containing the actives afoxolaner, fluralaner, lotilaner and sarolaner. Fipronil as a monotherapy has a slow SOK.
The isoxazoline group of drugs was launched in 2014 in the United States with afoxolaner. This was closely followed by oral fluralaner, sarolaner, topical fluralaner and lotilaner. While the precise labelling for each products differs in its license applications as far as spectrum of activity, minimum age of administration, method of administration, minimum body weight and dosing interval (Table 2), all of the products within this group are approved for treatment and prevention of flea infestations. The safety of all of these products and their ability to treat fleas has been well documented. Isoxazalines are absorbed systemically, the onset of action of all products is 2 to 4 hours with close to 100% of fleas killed within 8 hours (Datz, 2018).
Table 2. Licensed indications for each of the isoxazolines in the UK (monoformulations only) Source NOAH compendium August 2019.
| Drug | Formulation and mode of administration | Min age(weeks) | Min body wt(kg) | Dosing interval |
|---|---|---|---|---|
| Afoxolaner | Tablet (may be given with or without food) | 8 | 2.0 | Monthly |
| Fluralaner | Tablet (given at or around the time of feeding) | 8 | 2.0 | 12 weeks |
| Fluralaner | Spot-on (do not wash dog, immerse in water or swim within 3 days of application) | 8 | 2.0 | 12 weeks |
| Sarolaner | Tablet (may be given with or without food) | 8 | 1.3 | Monthly |
| Lotilaner | Tablet (given at or after the time of feeding) | 8 | 1.3 | Monthly |
Juvenoids
Two form of juvenoids or insect growth regulators (IGRs) are available for the management of flea infestations in the dog (Table 1). These are the chitin biosynthesis inhibitors (lufenuron) and the juvenile hormone mimetics (methoprene and pyriproxyfen). As products such as the isoxazolines and the nicotinic acetylcholine receptors agonists (NARAs) have a rapid and complete SOK before fleas can feed and lay eggs, hence breaking the life cycle, the use of juvenoids as part of a flea control programme may seem superfluous. However recent studies have shown a marked synergism between IGRs and adulticides (Rust and Hemsarth, 2019). It would seem that IGRs reduce the time required to control fleas within the environment and also lessen the likelihood that insecticide resistance may develop. Therefore current recommendations are to combine an adulticide with an IGR into any treatment regimen to increase the efficacy over the use of an adulticide alone (Rust, 2017).
Conclusion
The veterinary nurse is well placed to advise clients on the use of flea products. Many aspects should be considered when selecting a flea treatment. As well as the obvious factors such as safety and efficacy, things such as cost, ease of application and convenience are factors that can influence owner compliance, which is important in sustaining a flea control programme. Although many of the older products such as fipronil are still widely used and effective, more recent adulticides such as the NARAs and the isoxazalines have been shown to be safe, effective and convenient with a much more rapid SOK. SOK is important to reduce the flea burden on the animal, kill fleas before they can reproduce and rapidly reduce environmental infestation to bring skin disease under control quickly. However although the use of a rapid acting and persistent adulticide should form the mainstay of any treatment regimen, an integrated approach using an adulticide and an insect growth regulator has been shown to reduce flea numbers more quickly and effectively than the use of an adulticide alone. Therefore combination therapy with an adulticide and IGR should be prescribed for every dog as part of their routine flea control, to every dog to investigate pruritic skin disease and for every dog with an underlying problem of AD.
KEY POINTS
- Flea treatment is important in all pruritic canine skin disease.
- Good flea control should play an integral part of any treatment regimen for a dog with canine atopic dermatitis.
- Resistance to flea products is uncommon.
- Speed of kill is an important factor when choosing a flea adulticide.
- Any flea control programme should be integrated and not dependent on any one product.
- The veterinary nurse can play a key role in identifying fleas on pets and recommending appropriate treatment measures.


