The confidential enquiry into perioperative small animal fatalities states that small animal anaesthesia appears to be increasing in safety. However, greater patient care during the perianaesthetic period would reduce fatalities further (Brodbelt et al, 2008). While there is no direct evidence to prove that the use of capnography can reduce the risk of mortality, it has been shown to prevent morbidities and has allowed for the early detection of complications before significant physiological side effects are seen (Langton et al, 2010). This confirms Henao-Gurrero et al's (2005) opinion that capnography can improve the anaesthetic management of cases seen. Capnography is becoming more commonplace in the veterinary practice; however, only 37% of registered veterinary nurses questioned felt comfortable with its use (unpublished data).
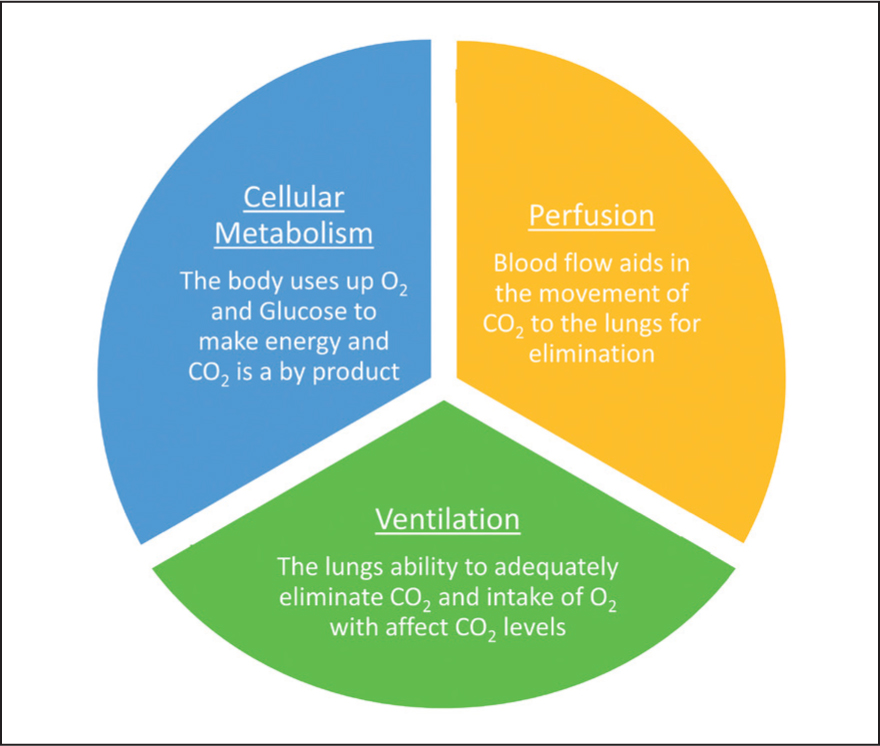
To understand capnography and the capnogram, there needs to be an understanding of how carbon dioxide (CO2) is regulated in the body and why it is important. CO2 in the body is determined by three factors:
- Cellular metabolism
- Perfusion
- Ventilation.
During cellular metabolism, inhaled oxygen (O2) passes from the lungs into the bloodstream via diffusion across a pressure gradient. O2 travels via the bloodstream around the body and is diffused into tissues. Here, the cells use O2 and glucose to produce energy and CO2 is created as a by-product of this. Metabolic rate can be affected by perfusion, oxygen diffusion, cell health, the temperature of the patient and thyroid function. This CO2 then enters the blood-stream and partially diffuses across the pressure gradient to be exhaled. This shows the importance that perfusion to the lungs and tissues has on the regulation of CO2 in the body. Therefore, normotension must be maintained during anaesthesia to aid CO2 regulation. While ventilation is often the primary focus when CO2 regulation is discussed, it is of equal importance to perfusion and cellular metabolism. Capnography not only informs about the patient's ventilatory status, but also provides information on systemic metabolism, perfusion and cardiac output (Marshall, 2004). Adequate ventilation depends on the respiratory rate as well as the volume of air within the breath (tidal volume). Therefore, hypoventilation can be caused by a reduction in respiratory rate, a reduction in tidal volume or both. Hypoventilation can have many causes, including the administration of respiratory depressive medications such as opioids, or in overweight patients where an increase in abdominal fat prevents the diaphragm from descending and contracting normally (Marshall, 2020). A summary of how CO2 is regulated in the body can be seen in Figure 1.
CO2 is transported around the body in three main forms:
- As carbonic acid after chemical reaction with water (60–70%)
- Bound to haemoglobin (20–30%)
- Dissolved in plasma (5–10%) (Wallace, 2021).
Carbonic acid goes through further chemical reactions to form bicarbonate and hydrogen ions. Hydrogen ions play a key role in acid–base regulation of the body, where increased hydrogen ions will increase the acidity of the blood.
It is important to remember that CO2 is the ventilatory driver of the body, and small changes in CO2 can encourage changes in either rate or depth of breaths (Simpson, 2015a). In contrast, only vast changes in O2 levels in the body would cause the body to respond. When CO2, and therefore hydrogen ions, increases in the blood, chemoreceptors in the blood, carotid and aortic bodies note this drop in pH and send nerve impulses to the respiration centre in the medulla oblongata and pons in the brain. The respiratory centre of the brain will then increase the respiratory rate to decrease CO2 levels and return blood pH to normal.
Prolonged exposure to both increased and decreased levels of CO2 in the blood can have negative side effects on the body which can be seen in Table 1.
Table 1. Negative side effects associated with hypo and hypercapnia
| Hypercapnia (PaO2 over 45 mmHg) | Hypocapnia (PaO2 under 35 mmHg) |
|---|---|
|
|
PaCO2 vs ETCO2
CO2 within the body can be measured either directly via arterial sampling, which gives us the partial pressure of CO2 in arterial blood (PaCO2), or indirectly via capnography. PaCO2 readings are considered the gold standard and are a more accurate representation of CO2 levels in the blood. PaCO2 levels should be between 35–45 mmHg. However, frequent arterial sampling is not always available, so capnography is used as an alternative. Capnography provides the end tidal CO2 (ETCO2) readings. It is said that PaCO2 will be 2–5 mmHg higher than ETCO2 because of normal volume perfusion mismatch within the lungs where not all sections of the lungs will be ventilating and perfused at the same time (Schauvliege, 2016). However, a study by Duke-Novakovski et al (2020) found a difference of 16.7–18.1 mmHg between PaCO2 and ETCO2 in rabbits when using sidestream and mainstream capnography. It should be noted that this study was conducted while using a breathing system with a high fresh gas flow rate, which could cause further dilution of the sample (Duke-Novakovski et al, 2020). In smaller patients, inaccurately low readings may be gained. This is evident in human medicine where, in paediatric patients under 2.5 kg, capnography readings were shown to be a particularly poor indicator of PaCO2 with ETCO2 being on average 13 mmHg lower than PaCO2 (Karlsson et al, 2017). While this may be a downfall of capnography, it does not mean that one should avoid its use in smaller patients. Users should be more cautious and be aware that an earlier intervention may be required when faced with ETCO2 readings that are on the higher end of normal.
Capnography
Capnography can provide the anaesthetist with a wealth of real-time information such as:
- Respiratory rate
- Estimation of PaCO2
- ETCO2
- Fraction of inspired CO2 (FiCO2)
- A non-invasive way of assessing systemic metabolism and cardiac output
- Effectiveness of cardiopulmonary resuscitation
- Effectiveness of ventilation, whether that be spontaneous or mechanical.
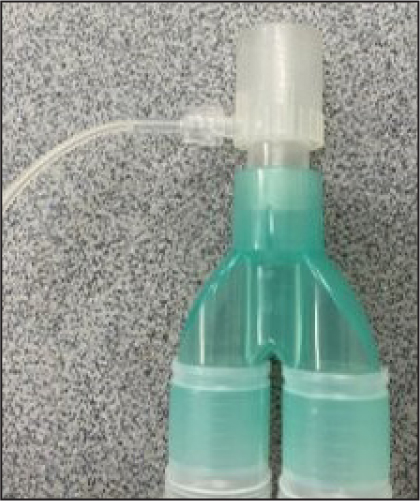
Most modern capnography works via infrared absorption spectrography, whereby CO2 absorbs infrared light. The infrared light is passed through the sample onto a sensor, the less infrared light the sensor picks up the higher the ETCO2 levels in the sample and vice versa. Therefore, capnography measures the concentration of CO2 in expired gas (Bagshaw-Wright, 2018) and importantly is a monitoring tool for ventilation rather than oxygenation (Maclennan and McCurry, 2020). The levels of CO2 expired are then plotted on a graph to give the capnogram. Both mainstream and sidestream capnography are available, however, sidestream is more commonly seen in practice. An example of sidestream capnography can be seen in Figure 2. When using sidestream capnography a small sample is constantly being drawn into the machine – this can be as much as 200–300 ml/min (McMillan, 2017). For smaller patients, this can be more than their tidal volume; this means the machine will provide inaccurately low readings which are being diluted by other gases in the breathing system.
Caring for your capnography
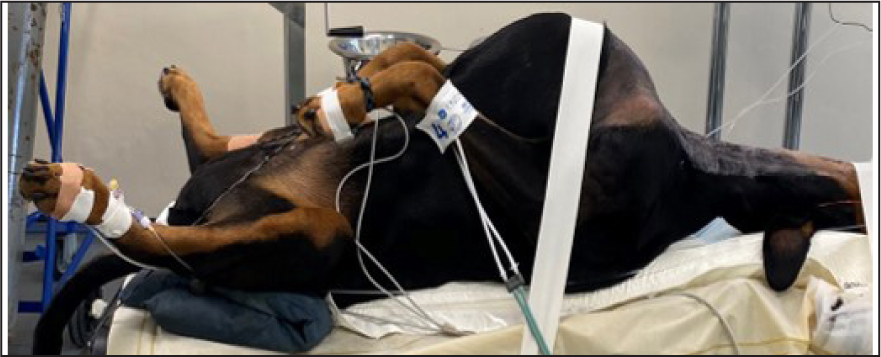
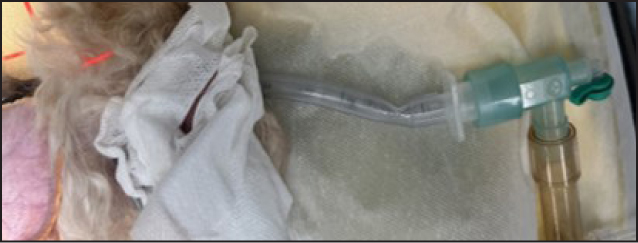
The monitoring device must be cared for appropriately and according to the manufacturer's guidelines to ensure its accuracy. Sampling lines should be checked regularly and if there are signs of water or fluid damage these should be exchanged for new lines. Fluid may build up in the sampling line during long anaesthetics where there is a build-up of condensation in the line, as seen in Figure 3. Excessive fluid in the sampling line may lead to inaccurate readings. Water traps, which can be found on the side of many monitors, should be regularly emptied. If fluid is allowed to build up in these, it can enter the monitor and cause water damage to the machine. A partially full water trap after one 4-hour anaesthetic on a large breed dog can be seen in Figure 4. The monitor and sampling lines should be checked for any damage before induction of anaesthesia as part of the safety checklist (Association of Veterinary Anaesthetists, 2023). Lines are commonly stood on which can cause cracks as seen in Figure 5. This can cause leaks which cause environmental pollution and inaccurate readings.



The capnogram
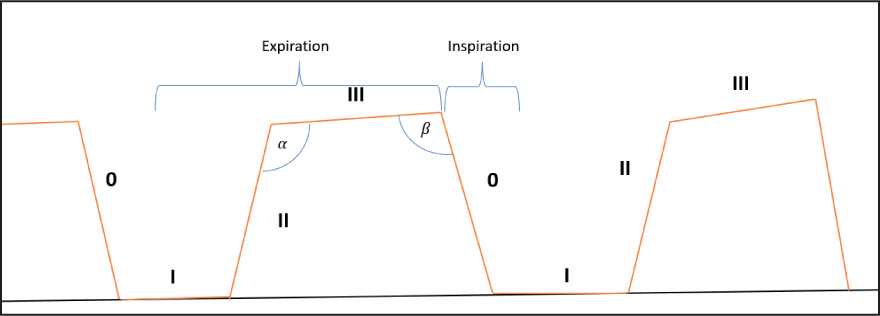
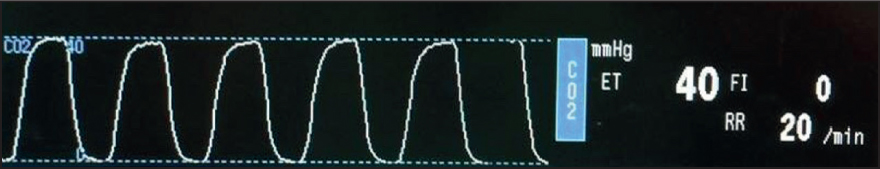
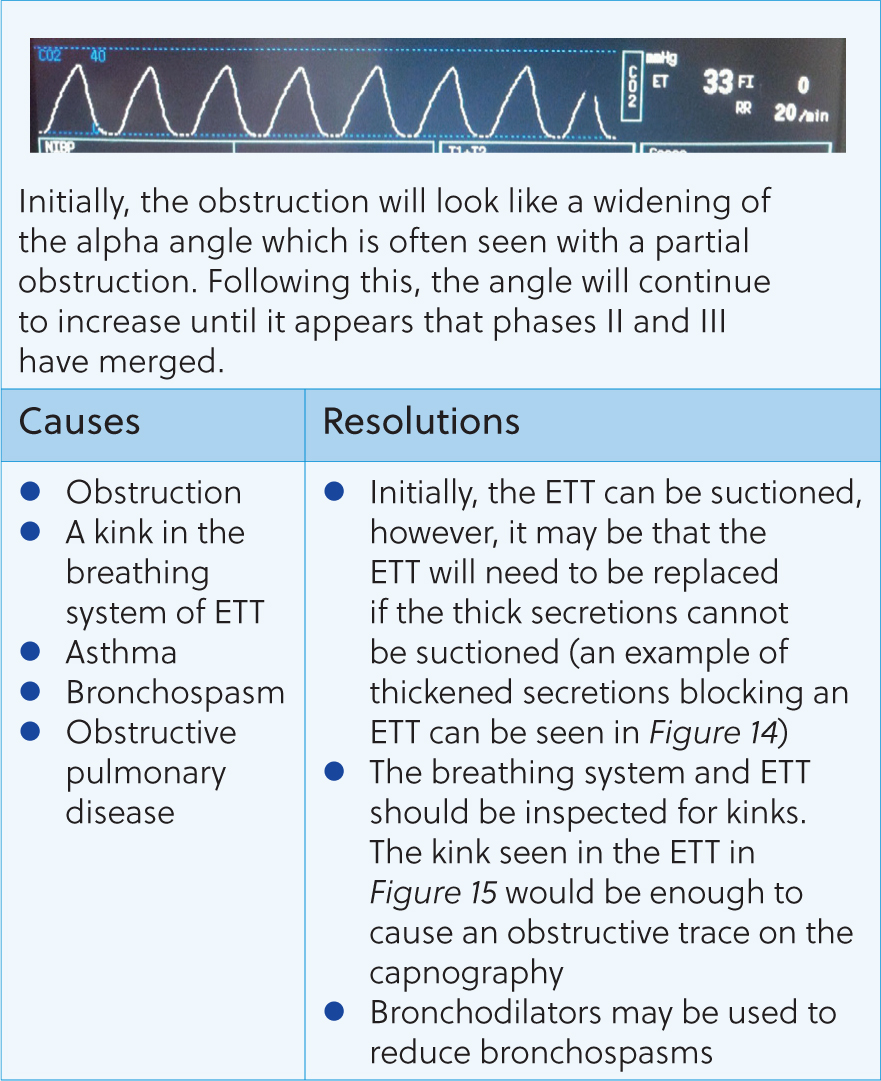
The standard capnogram should resemble the ones seen in Figures 6 and 7. The capnogram is represented by four phases – three expiratory and one inspiratory phase (Simpson, 2015b). Phase 0 is the inspiratory downstroke and contains CO2-free gas. Phase I is the expiratory baseline which should ideally be zero – this is the start of exhalation (Clarke et al, 2014). Phase II is the expiratory upstroke and shows rapidly increasing CO2 levels – this is a mixture of anatomical dead space and alveolar gas (Clarke et al, 2014). Phase III represents the expiratory plateau – here, ETCO2 levels will raise slightly until it reaches its maximum for that breath (end-tidal value) (Angell, 2023). This upward slope is due to the continuing CO2 production and emptying of alveolar units with the basal lung units emptying last (Haskins, 2015). On the author's capnogram, there are also alpha and beta angles at either end of phase III. The alpha angle will increase when there is a partial airway obstruction or when positive end-expiratory pressure is applied. The beta angle will increase when there is rebreathing of carbon dioxide, therefore these can both be deployed for early detection of these complications.
Troubleshooting capnography
When troubleshooting capnography, the following should always be considered:
- Is the trace interpretable (for example, is the patient being moved by the surgeon which can look like tachypnoea)?
- Is the patient's endotracheal tube (ETT) in the trachea and sufficiently inflated?
- Is the patient sufficiently anaesthetised and have they received adequate analgesia?
- What is the patient's blood pressure and temperature?
- Is the fresh gas flow adequate (if using a non-rebreathing system)?
- Is the CO2 absorber exhausted (if using a rebreathing system)?
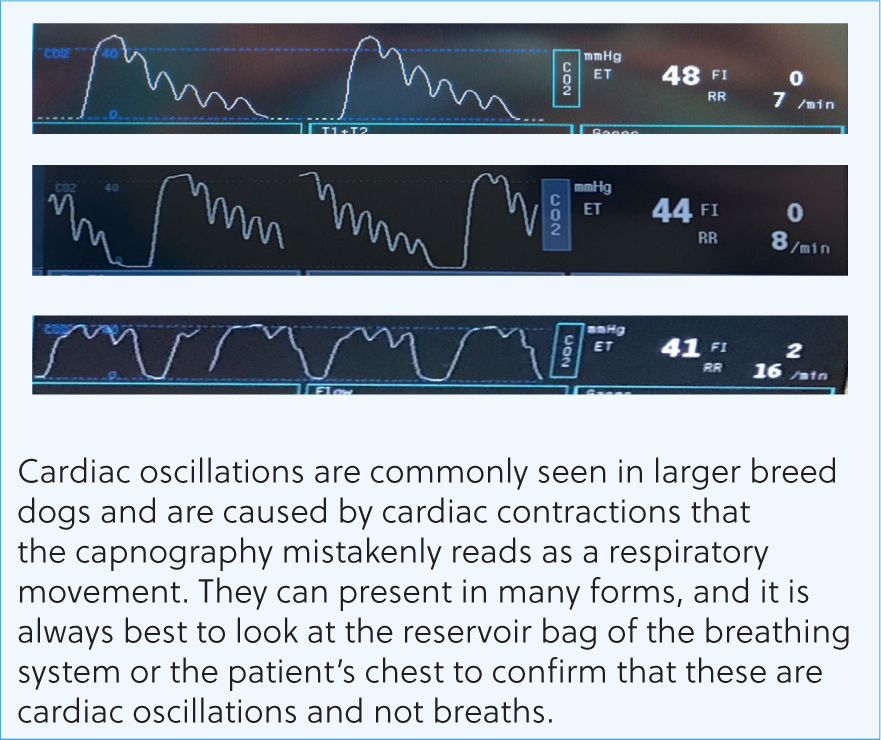
Following these questions can help guide the anaesthetist to the issue and how to resolve it. Figures 8–16 contain the most common abnormal capnography traces and problems that will be seen, possible causes and how to resolve these.
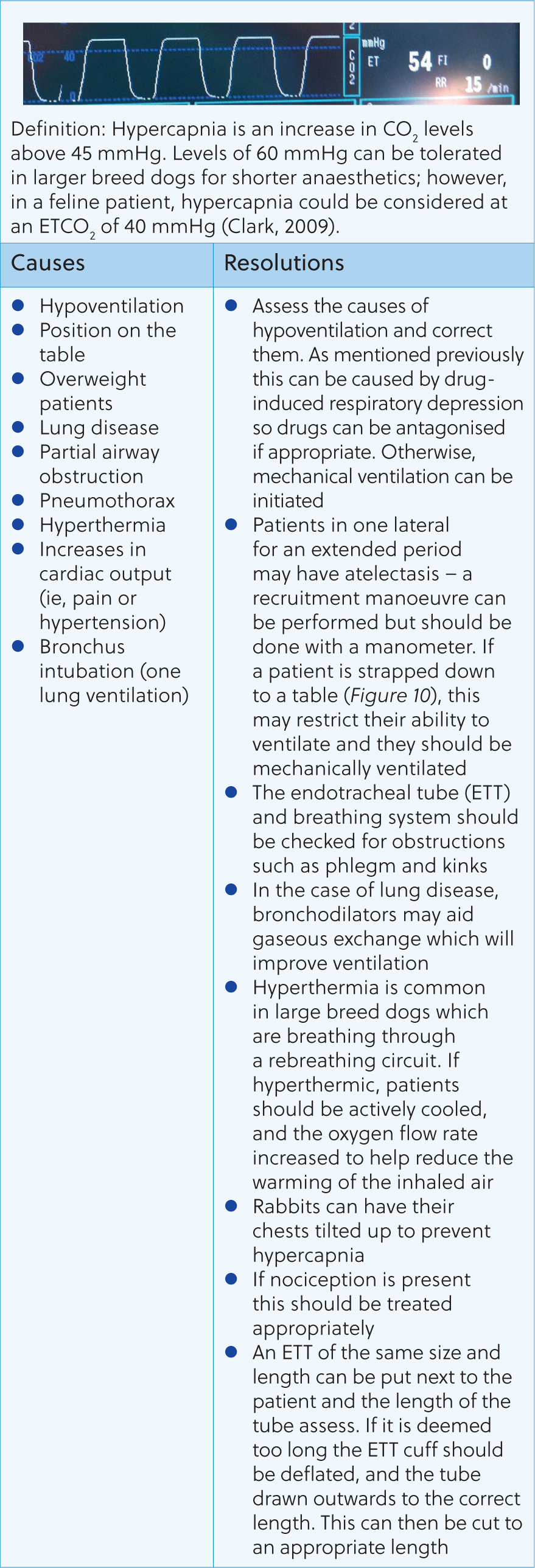
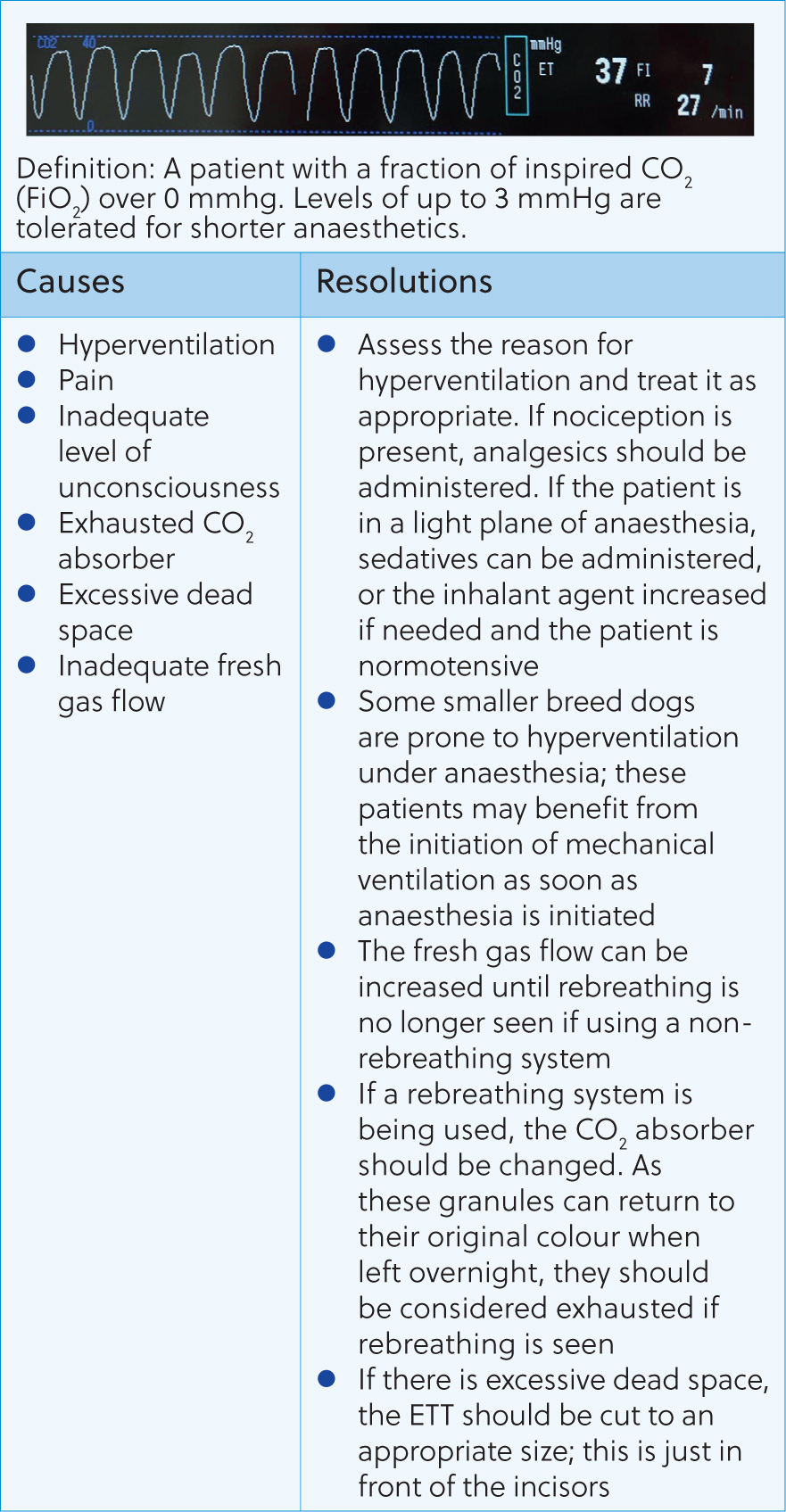
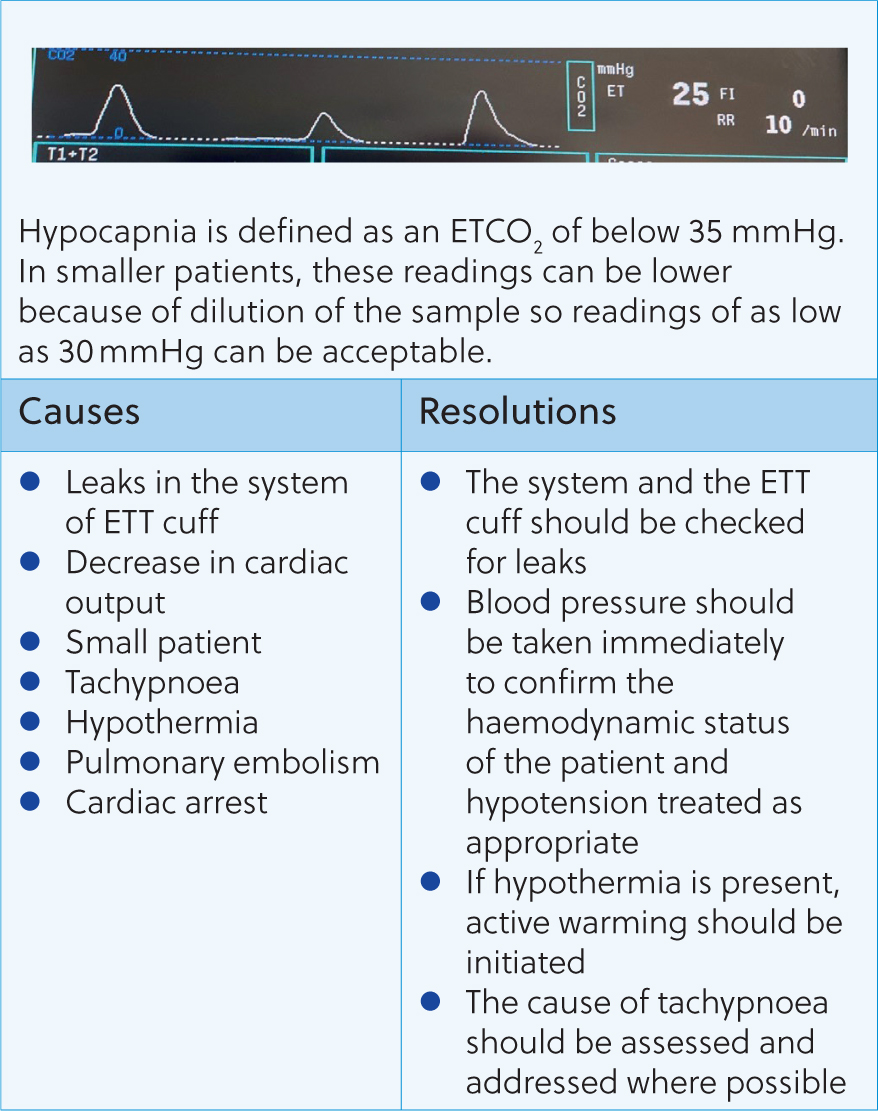


Capnography can be an invaluable tool for the anaesthetist. Early detection and correction of ventilatory issues can reduce the risk of mortality and help to provide safer anaesthesia. However, many nurses can feel unconfident in their ability to assess and react to changes seen. This article shows that a simple, systematic approach, coupled with an understanding of CO2 regulation in the body, can help the registered veterinary nurse understand and react appropriately to capnography traces.
KEY POINTS
- The veterinary nurse needs to be aware of normal respiratory physiology in order to interpret and react to changes seen on the capnogram.
- Capnography is an invaluable monitoring tool during anaesthesia and provides us with an indirect way of measuring cellular metabolism, ventilation and perfusion.
- In order to get the most out of the capnogram as a monitoring tool they need to be cared for as per the manufacturer's guidelines.
- The veterinary nurse should be aware of how the size of the patient and the breathing system used can affect normal EtCO2 readings.


